Article
SGLT-2 Inhibitors Aid the Management of Complex Cases of Type 2 Diabetes
Author(s):
A growing class of medications, SGLT-2 inhibitors have helped physicians manage otherwise uncontrolled cases of type 2 diabetes.
The results of some epidemiological studies estimate that by 2050, 1 in 3 individuals in the United States will have type 2 diabetes (T2D), and disease proliferation means that the incidence of commonly occurring comorbidities like cardiovascular disease (CVD), microvascular complications, hypertension, hyperlipidemia, and kidney disease will likewise increase.
As a result, effectively addressing individual patient needs will continue to become more challenging when attempting to manage hyperglycemia and achieve hemoglobin A1C (A1C) targets and slow progressive degeneration of pancreatic β cells. Compounding these challenges is the necessity to strike a balance in care to avoid hypoglycemia, manage existing comorbidities, and prevent new complications.
When patients have difficulty reaching and maintaining their A1C targets, physicians can add a second oral antihyperglycemic agent as a combined therapy approach to better manage hyperglycemia. As always, the effectiveness depends on the patient’s individual case, lifestyle habits, and adherence to the protocol and an additional antihyperglycemic agent carries the potential for a greater risk of hypoglycemia.
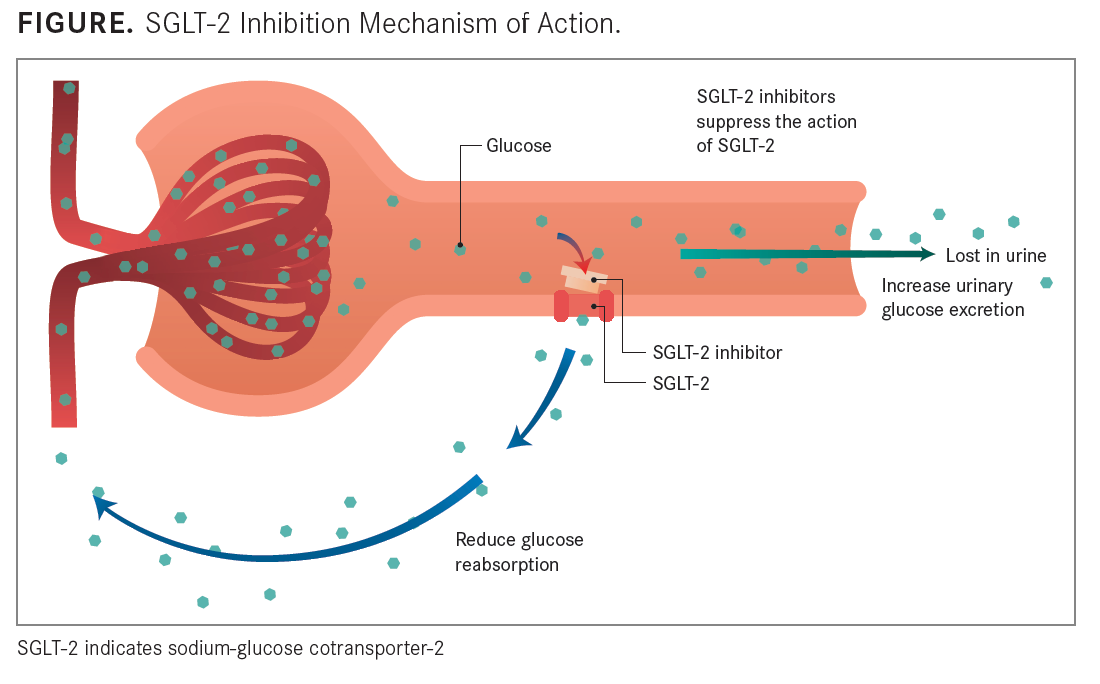
FIGURE
A newer class of oral antihyperglycemic drugs, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, have a β cell-independent mechanism of action (See ). These therapies hinder the SGLT-2 protein in the proximal convoluted tubule leading from Bowman capsule, where 90% of glucose reabsorption takes place. Once that reabsorption is prevented, glucose is excreted in the urine, and because of this mechanism of action, it is possible that SGLT-2 inhibitors promote increased insulin sensitivity and can, therefore, be used in tandem with any currently approved oral and injectable antihyperglycemic agents.
“SGLT-2 inhibitors have revolutionized care for patients with T2D,” Willa Hsueh, MD, told MD Magazine. “I think they are an easy way to lower glucose if patients can deal with 2 common [adverse] effects: having to get up all the time at night [because of] increased frequency of urination and possibly getting yeast infections, in women. [But] the vast majority tolerate them well. When [patients] see their glucose levels decrease and their A1C go down, they’re really encouraged.”
Hsueh, the director of the Diabetes and Metabolism Research Center at The Ohio State University’s Wexner Medical Center, noted that additionally, these inhibitors work well with other medicines—most commonly metformin.
Because SGLT-2 inhibitors operate independently from insulin and do not stimulate insulin secretion, there is a lower risk of hypoglycemia than with certain other oral antihyperglycemic agents, particularly sulfonylureas. SGLT-2 inhibitors may also promote weight loss and reduce the complications brought on by markers of metabolic syndrome.
“For cardiovascular prevention, certain SGLT-2 inhibitors and glucagon-like peptide-1 [GLP-1] receptor agonists have been shown to reduce cardiovascular events,” Deepak L. Bhatt, MD, MPH, the executive director of interventional cardiovascular programs at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, told MD Magazine. “While lifestyle modification would still be essential, and metformin will likely remain first-line therapy [because of] low cost and familiarity, the newer drugs from these classes appear to be a significant advance, though their expense [limits their] use, to an extent.
“Putting expense aside, the data would support their use as second agents after metformin in diabetic patients with established cardiovascular disease,” he added.
With several pathways for cardiovascular risk in T2D, CVD remains the most common cause of mortality in the diabetic population. Results from studies have shown that a 1% increase in A1C is associated with a 21% increase in cardiovascular events, including myocardial infarction.1 This underscores the complex, multifactorial pathophysiological link between T2D and CVD and the necessity for aggressive treatment for patients who are at higher risk.
“There are now 2—some would say 3—medication categories that have been proved to reduce adverse cardiovascular sequelae of diabetes: the SGLT-2 inhibitors and the GLP-1 receptor agonists,” Silvio Inzucchi, MD, the medical director of the Yale Medicine Diabetes Center and a professor of medicine at Yale School of Medicine, told MD Magazine®. “The thiazolidinediones can probably be put in the same category. These drugs should be favored in those with T2D and established CVD, especially empagliflozin and liraglutide, which have been shown to reduce cardiovascular mortality as well. This is now so stipulated by the [American Diabetes Association] in its most recent Standards of Care.”
Inzucchi, who is also the clinical director of the section of endocrinology at Yale, noted that with the SGLT-2 inhibitor class, empagliflozin and canagliflozin lead the way, while liraglutide and semaglutide are some of the best-in-class of the GLP-1 receptor agonists. He highlighted pioglitazone as a top choice for thiazolidinediones but acknowledged that the data supporting the therapy came from a study involving patients with insulin resistance and not diabetes.
Along with managing A1C, it is critical to monitor and effectively attend to such commonly occurring complications as microvascular disease and hypertension early in the disease course if possible, as they may be precursors to macrovascular disease and CVD.
“The SGLT-2 inhibitors are the glucose-lowering class that [have] the best effect on blood pressure, so this may be an added bonus of using these agents,” Inzucchi said. “For nephropathy, I believe the data support that the best agents may be the SGLT-2 inhibitors, which appear to have a mechanism-specific effect on reducing glomerular stress and therefore improve renal outcomes long term. Sometimes acute drops in glucose can cause paradoxical worsening of retinopathy or neuropathy, especially retinopathy.
“Semaglutide was in fact associated with more retinopathy, but the data I’ve seen presented suggest that this may be the result of more rapid lowering of glucose in those with preexisting retinopathy,” he added.
Selecting appropriate individual A1C targets on a case-by-case basis, dependent on the stage of diabetes and number of complications, can lead to better outcomes for overall patient health and management of hyperglycemia, as well as more effective treatment with combined therapies.
“I aim for a goal of 6.0% to 6.5% A1C in patients with early T2D with no complications and 7.5% to 8.0% A1C in patients with the disease that’s difficult to manage with a lot of complications,” Hsueh said. “You don’t want a lot of hypoglycemia in those patients. You need to look at kidney function because that’s also an important consideration in what you start. I prefer to give drugs [such as SGLT-2 inhibitors and GLP-1 receptor agonists] that don’t cause hypoglycemia first. So once you decide where you are, if your goal is 6.5% A1C and your patient is at 8.0% A1C, you might start 2 drugs at once, plus lifestyle modifications.”
Hsueh continued that patients need to be targeted in the prediabetic state, prior to the onset of any complications. “I would like to see that prediabetes is not a nondisease state,” she said.
She said that as many as 20% of patients with prediabetes also have microalbuminuria, and another 20% have been reported to have some kind of retinopathy. Most notably, though, more than 30% have been reported to have neuropathy changes “when you actually measure them with nerve-conduction tests,” Hsueh said.
“I think you really need to identify those patients—at least do an eye exam and look for microalbuminuria, as well as the regular chemistry panels, and be more aggressive about weight loss and better eating habits,” she added.
Hsueh advocates conducting studies on patients in the prediabetic state to determine what kind of complications they might expect, based on any markers of metabolic syndrome, while there is opportunity to prevent disease complications and protect the islet cells with early intervention.
“Compared to a normal person, prediabetics have lost almost 50% of their islet cells, and if you compare [them] to a person with frank diabetes, they’ve lost 90% of their islet cells,” Hsueh said. “So if you say prediabetes is not a bad state—it is a bad state. We need to find these patients early, and they’re more likely to be found in primary care offices than in the endo offices. We ought to have a statement on how we should be evaluating these patients and what we should be doing.”
The efficacy of any pharmacologic intervention depends heavily on the patient’s guidance for and compliance with lifestyle modifications. It follows that “small” improvements in lifestyle habits could lead to large improvements in disease course and migration of complications. Tailoring a diet and exercise regimen to a patient’s individual needs is critical to long-term care and effective, consistent management of hyperglycemia.
“Coupled with the drugs, I think we could make a big dent in the increasing incidence of T2D with adherence to individualized diet and exercise regimens,” said Hsueh. “The good news is that if they lose weight and they change their lifestyle, they can come from a prediabetic to a normal state; they can revert to normal, and that would be the goal. There are ways we can recognize prediabetes: a strong family history and having many components of metabolic syndrome and obesity. There’s a whole bunch of things you can do.
“The more they tip toward the risk side, the more you need to intervene,” Hsueh concluded.
REFERENCE
Selvin E, Marinopoulos S, Berkenblit G, et. al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421-431.



