Article
FDA: Surgery on Babies Risks Brain Damage
Author(s):
After a review of existing studies, the FDA today sent out a warning that surgery lasting three hours or more poses a threat of brain or other neurological damage in babies, fetuses, and toddlers.
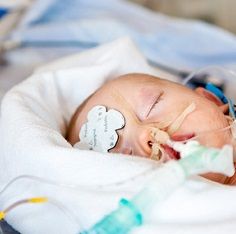
Long surgeries can cause neurological damage in babies, fetuses, and toddlers.
When infants, young children, or pregnant women need surgery the risk of brain or neurologic injury from anesthesia has long been a concern.
In a Drug Safety Communication sent today the US Food and Drug Administration (FDA) said that anesthesia administered for more than three hours may adversely affect the developing brains of children younger than 3 years old and the fetus in pregnancies that are in the third trimester. The same is true for repeated surgeries.
That includes 11 drugs that block N-methyl-D-aspartate receptors or potentiate gamma-aminobutyric acid activity, or both.
“No specific medications have been shown to be safer than any other” the FDA noted.
“We recognize that in many cases these exposures may be medically necessary and these new data regarding the potential harms must be carefully weighed against the risk of not performing a specific medical procedure,” Janet Woodcock, MD, director of the agency’s Center for Drug Evaluation and Research said in a statement accompanying the safety announcement.
While some common procedures like hernia repair generally take far less than three hours, open heart procedures can last up to eight hours.
The warning is based on an FDA evaluation of published studies in pregnant animals and young animals. All showed loss of nerve cells in the brain or effects in the animals’ behavior or ability to learn.
In another study published in 2015, Australian researchers looked at results in infants in 28 hospitals seven nations, including the US, who had anesthesia for less than an hour during hernia repair surgery.
The children were assessed two years after the surgery to see if there were adverse neurodevelopmental outcomes.
They found they showed no such signs, but the results were taken as not useful for weighing the risks in longer procedures.
That research group is planning to follow their study group through five years post-surgery.
The full document “FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women” lists the drugs and has links to studies.
Related Coverage:





