Article
The Benefits of a Multimodal Strategy Using Opioid-Reducing Approaches in Postsurgical Pain Management
Content of this article was sponsored by Pacira BioSciences, Inc. Copyright 2022 and published by HCPLive®.
More than 73 million patients undergo surgical procedures each year in the United States. The majority experience suboptimal pain management following surgery, and, particularly for patients who receive a major procedure (eg, colorectal surgery), the pain can be severe, heighten the stress response, and compromise the restoration of function.1-3 Of additional concern are surgical patients who receive opioids (eg, morphine sulphate, fentanyl citrate, meperidine hydrochloride) that, according to surgical recovery guidelines, should be used minimally for pain relief.1,3-5 Although opioids can effectively reduce pain following surgery, they are associated with adverse events (AEs), such as respiratory and/or central nervous system depression, nausea or vomiting, and pruritus.4,6 Their use may lengthen hospital stays and increase both total hospital costs and the incidence of readmissions.7 In addition, up to 92% of patients report having unused opioids after both inpatient and outpatient surgery; these unused opioids can lead to opportunities for nonmedical use, accidental exposure, overdose, and potentially increasing new cases of opioid addiction. As a result, 136 people die every day from an opioid-related overdose in the United States.8-10 This current environment necessitates the implementation of improved pain management strategies to enhance outcomes, particularly for major procedures, and spare the use of opioids.
Enhanced Recovery after Surgery® (ERAS®) protocols involve patient-centered, multidisciplinary care to help reduce the stress response following surgery and to optimize recovery.3 Regarding ERAS protocols for colorectal surgery specifically, recommendations in the 2017 clinical practice guidelines from the American Society of Colon and Rectal Surgeons (ASCRS) and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) include implementation of a multimodal, opioid-sparing pain management plan prior to anesthesia administration. This approach can include scheduled dosing of oral narcotic alternatives, such as nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen, to reduce dependence on opioids for pain relief. Additional analgesics (ie, gabapentinoids, ketamine, and α2-adrenoreceptor agonists) may also be used, although associated AEs (eg, dizziness, sedation) may slow recovery following surgery. For open colorectal surgery, thoracic epidural analgesia is considered the mainstay of pain management; however, it is associated with a failure rate of up to 32%, and it may hinder recovery when used in laparoscopic surgery. The ASCRS/SAGES guidelines state that local anesthetics, such as EXPAREL® (bupivacaine liposome injectable suspension), that are administered via wound infiltration or transverse abdominis plane (TAP) block may effectively manage pain associated with both open and laparoscopic colorectal surgery. TAP blocks may achieve improved results when administered prior to, rather than toward the end of, surgery induction.5,11
In general surgery, use of ERAS protocols with multimodal pain management has improved outcomes; these have included lower postsurgical pain scores, fewer complications, lessened postsurgical fatigue, quicker mobilization, better function, and higher patient satisfaction.8,12-14 Use of ERAS protocols also may shorten the length of hospital stays and produce lower readmission rates and overall costs.13,14 In addition, benefits of possible diminished opioid use include fewer AEs; lower mortality rates, number of readmissions, and costs; shorter hospital stays; and reduced availability of opioids for nonmedical use and associated addiction and/or overdose.4,8
EXPAREL as Part of a Multimodal Approach to Postsurgical Pain Management
EXPAREL, the only single-dose, long-acting analgesic that offers a nonopioid option for postsurgical pain control, can play an important role in a multimodal approach. It is indicated in patients aged 6 years and older for single-dose infiltration to produce postsurgical local analgesia; in adults, it is indicated as an interscalene brachial plexus nerve block to produce postsurgical regional analgesia.11,15 EXPAREL is composed of a suspension with proprietary, biocompatible multivesicular liposomes that contain bupivacaine; this drug is released slowly in a consistent, controlled manner to maintain plasma levels safely below cardiac and neurotoxic thresholds (2000-4000 ng/mL) while providing local analgesia for up to 5 days.11,15-17 In addition to effectively managing postsurgical pain, EXPAREL spares the use of opioids and reduces the need for the pumps and catheters often used for prolonged analgesia; use of these devices can involve a greater investment of time and resources, high doses of local anesthetics, and increased risk of user error and of complications associated with potential infection or device failure.17
Dosing and administration
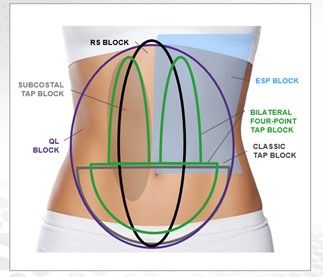
EXPAREL is available as a single-dose vial containing either 266 mg (20 mL) or 133 mg (10 mL) of bupivacaine liposome injectable suspension.11 For local infiltration, EXPAREL flexible dosing allows for tailoring for each patient based on the size of the surgical area, the volume of medication needed to cover the area, specific patient factors that could compromise safety with use of the drug, and patient age (ie, pediatric vs adult). The maximum dose should not exceed 266 mg. For regional analgesia via interscalene brachial plexus nerve block in adults, the recommended dose is 133 mg.11 Regional anesthesia is achieved through a variety of blocks, such as classic TAP block, subcostal TAP block, bilateral 4-point TAP block, rectus sheath block, quadratus lumborum block, and erector spinae plane block (Figure).18-21
Figure. EXPAREL in Abdominal Field Blocks18-21
ESP, erector spinae plane; QL, quadratus lumborum; RS, rectum sheath; TAP, transversus abdominis plane.
Administration volume and technique are critical to achieve optimal results. Bupivacaine hydrochloride diffuses into tissues differently than does EXPAREL; therefore, EXPAREL should be administered in a different manner. EXPAREL stays where placed more precisely and requires more injections and higher volume to effectively cover the surgical area. It may be administered undiluted, or it can be diluted with 0.9% saline or lactated Ringer solution to increase the volume to a final concentration of 0.89 mg/mL.11 Bupivacaine hydrochloride may be administered immediately before EXPAREL or combined in a syringe with EXPAREL for simultaneous administration, provided that the milligram dose ratio of bupivacaine hydrochloride solution to EXPAREL is not greater than 1:2.11
Clinical Benefits With EXPAREL in Laparoscopic and Open Colorectal Surgery
Pain following major abdominal procedures (eg, colorectal surgery) typically is very high, and it must be effectively managed to decrease the stress response and facilitate the recovery process.3 When used as part of a multimodal approach, EXPAREL can effectively provide local or regional analgesia in colorectal surgery and improve overall outcomes, as demonstrated by results from several clinical trials.22-24
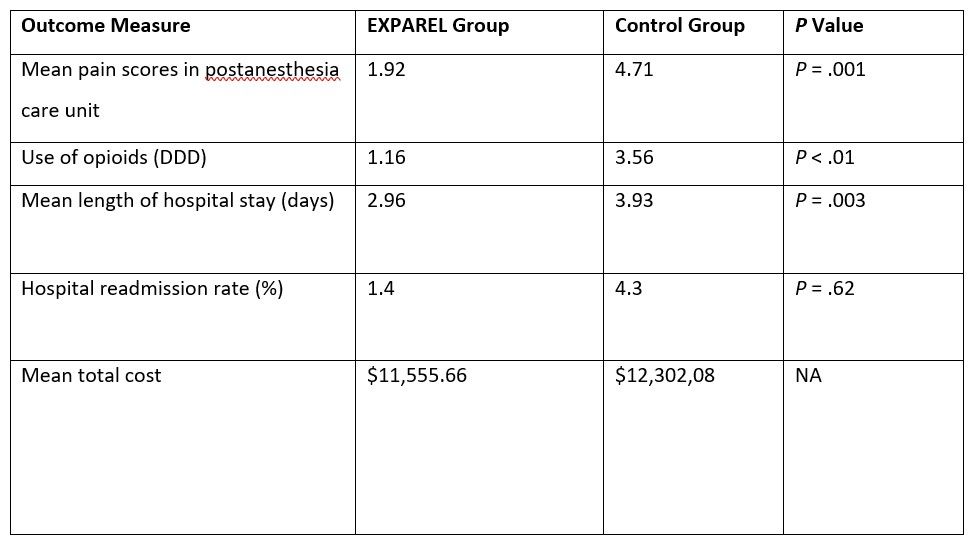
Outcomes from a retrospective, single-center study on EXPAREL administered via local wound infiltration to patients undergoing laparoscopic colorectal surgery showed improvements in pain, hospital length of stay, readmission rates, costs, and use of opioids.22 A prospective cohort placed on an ERAS protocol that included EXPAREL (n = 70) was compared with a historical cohort of patients who were placed on an ERAS protocol alone (n = 70).22 At the end of the surgical procedure, patients in the experimental group received EXPAREL, 266 mg expanded with 20 mL normal saline, and 20 mL of bupivacaine hydrochloride, 0.25%, which was injected via local wound infiltration at the laparoscopic port site. Across both cohorts, the mean operation duration and mean final incision lengths were similar. All patients were on a standard ERAS that included alvimopan given prior to surgery and throughout the entire hospital stay, minimal opioids given around the time of surgery, glucocorticosteroids, nonopioids given postsurgery, and early oral pain relief.22 In the postanesthesia care unit, patients in the EXPAREL group experienced significantly lower mean pain scores than the control group and significantly lessened use of opioids as measured by the World Health Organization’s assumed average maintenance dose per day (defined daily dose [DDD]) (Table 1).22 Pain medication consumption on postsurgery days 0, 1, 2, and 3 was consistently lower in the EXPAREL group than it was in the control group.22 In addition, the mean length of hospital stay was significantly shorter and the readmission rate lower for the EXPAREL group than for the control group, respectively. Mean total costs were also lower for each patient who received EXPAREL ($11,555.66) than for those who did not ($12,302.08) (Table 1).22
Table 1. Post-surgical Pain Management for Laparoscopic Colorectal Surgery: Results with EXPAREL Administered Via Local Wound Infiltration versus Control22
DDD, World Health Organization’s defined daily dose; NA, not applicable
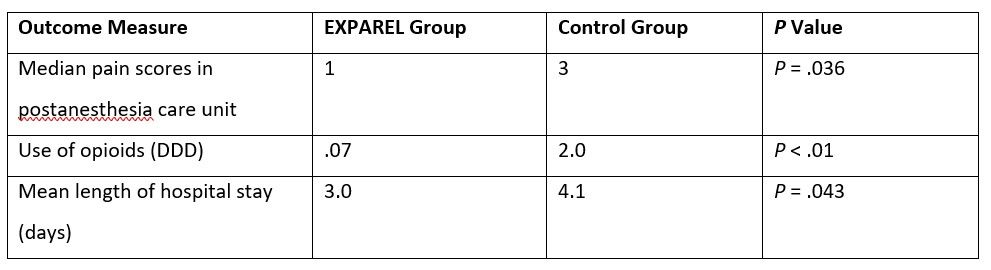
In another prospective cohort study, the use of EXPAREL administered via both local wound infiltration and TAP block was associated with similar results among patients who underwent elective minimally invasive colorectal resection and who followed a standard ERAS protocol. Patients in the experimental group (n = 25) received EXPAREL (266 mg expanded with 30 mL of bupivacaine hydrochloride, 0.25%, and 30 mL of normal saline); 40 mL was administered as a bilateral TAP block (20 mL per side) prior to surgical incision, and 40 mL was injected as a local infiltration around the peritoneum at port/extraction sites. The control group did not have a TAP block or local wound infiltration and was treated according to the standard ERAS protocol. Patients given EXPAREL experienced significantly lower final pain scores in the postanesthesia care unit than did those in the control group, lessened mean opioid consumption on postoperative day zero, and shorter mean length of stay (Table 2).23
Table 2. Post-surgical Pain Management for Colorectal Resection: Results with EXPAREL Administered Via Local Wound Infiltration and TAP Block versus Control22
DDD, World Health Organization’s defined daily dose.
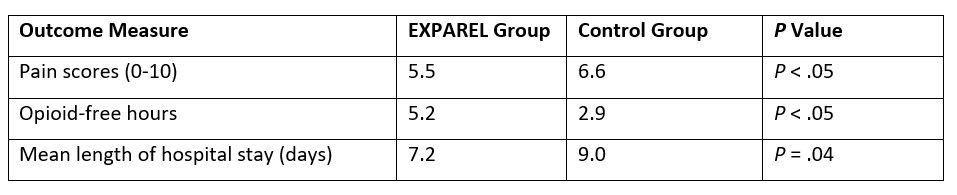
Comparable outcomes have also been achieved with use of EXPAREL in major open colorectal surgery, as demonstrated by results from a retrospective study of 66 patients who received multimodal pain management using EXPAREL, 266 mg given via local wound infiltration, compared with 167 patients given conventional pain management strategies (ie, intravenous opioids) for both open and laparoscopic colorectal surgeries. Postoperative pain scores (scale, 0-10) were lower for the EXPAREL group than for the conventional pain management group; a greater number of opioid-free hours and shorter hospital stay also were noted (Table 3).24
Table 3. Post-surgical Pain Management for Open and Laparoscopic Colorectal Surgery: Results with EXPAREL Administered Via Local Wound Infiltration versus Control24
Conclusions
Use of an opioid-sparing multimodal regimen can reduce opioid-related AEs and improve overall outcomes following surgery.4,8,24 As part of a postsurgical pain management regimen, EXPAREL is a compelling long-acting, nonopioid option to help manage postsurgical pain with fewer opioids.17 It offers administration versatility with both local infiltration and regional blocks among patients aged 6 years and older and effectively achieves analgesia across both laparoscopic and open surgeries.11,15,22-24
Important Safety Information
Indication
EXPAREL is indicated for single-dose infiltration in patients aged 6 years and older to produce postsurgical local analgesia and in adults as an interscalene brachial plexus nerve block to produce postsurgical regional analgesia. Safety and efficacy have not been established in other nerve blocks.
Important Safety Information
- EXPAREL is contraindicated in obstetric paracervical block anesthesia.
- Adverse reactions reported in adults with an incidence greater than or equal to 10% following EXPAREL administration via infiltration were nausea, constipation, and vomiting; adverse reactions reported in adults with an incidence greater than or equal to 10% following EXPAREL administration via interscalene brachial plexus nerve block were nausea, pyrexia, and constipation.
- Adverse reactions with an incidence greater than or equal to 10% following EXPAREL administration via infiltration in pediatric patients aged 6 years to less than 17 years were nausea, vomiting, constipation, hypotension, anemia, muscle twitching, vision blurred, pruritis, and tachycardia.
- If EXPAREL and other nonbupivacaine local anesthetics, including lidocaine, are administered at the same site, there may be an immediate release of bupivacaine from EXPAREL. Therefore, EXPAREL may be administered to the same site 20 minutes after injecting lidocaine.
- EXPAREL is not recommended for use in the following patient populations: patients less than 6 years old for infiltration, patients less than 18 years old for interscalene brachial plexus nerve block, and/or pregnant patients.
- Because amide-type local anesthetics, such as bupivacaine, are metabolized by the liver, EXPAREL should be used cautiously in patients with hepatic disease.
Warnings and Precautions Specific to EXPAREL
- Avoid additional use of local anesthetics within 96 hours following administration of EXPAREL.
- EXPAREL is not recommended for the following types or routes of administration: epidural use, intrathecal use, regional nerve blocks other than interscalene brachial plexus nerve block, or intravascular or intra-articular use.
- The potential sensory and/or motor loss with EXPAREL is temporary and varies in degree and duration depending upon the site of injection and dosage administered; it may last for up to 5 days, as seen in clinical trials.
Warnings and Precautions for Bupivacaine-Containing Products
- Central Nervous System (CNS) Reactions: There have been reports of adverse neurologic reactions with the use of local anesthetics. These include persistent anesthesia and paresthesia. CNS reactions are characterized by excitation and/or depression.
- Cardiovascular System Reactions: Toxic blood concentrations depress cardiac conductivity and excitability, which may lead to dysrhythmias, sometimes leading to death.
- Allergic Reactions: Allergic-type reactions (eg, anaphylaxis and angioedema) are rare and may occur as a result of hypersensitivity to the local anesthetic or to other formulation ingredients.
- Chondrolysis: There have been reports of chondrolysis (mostly in the shoulder joint) following intra-articular infusion of local anesthetics, which is an unapproved use.
- Methemoglobinemia: Cases of methemoglobinemia have been reported with local anesthetic use.
Full Prescribing Information is available at www.EXPAREL.com
References
1. Kessler ER, Shah M, Gruschkus SK, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33(4):383-391. doi:10.1002/phar.1223
2. Joshi GP, Schug SA, Kehlet H. Procedure-specific pain management and outcome strategies. Best Pract Res Clin Anaesthesiol. 2014;28(2):191-201. doi:10.1016/j.bpa.2014.03.005
3. McEvoy MD, Scott MJ, Gordon DB, et al; Perioperative Quality Initiative (POQI) I Workgroup. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on optimal analgesia within an enhanced recovery pathway for colorectal surgery: part 1-from the preoperative period to PACU. Perioper Med (Lond). 2017;6:8. doi:10.1186/s13741-017-0064-5
4. Shafi S, Collinsworth AW, Copeland LA, et al. Association of opioid-related adverse drug events with clinical and cost outcomes among surgical patients in a large integrated health care delivery system. JAMA Surg. 2018;153(8):757-763. doi:10.1001/jamasurg.2018.1039
5. Carmichael JC, Keller DS, Baldini G, et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum. 2017;60(8):761-784. doi:10.1097/DCR.0000000000000883
6. Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159-180. doi:10.1054/jpai.2002.123652
7. Gan TJ, Robinson SB, Oderda GM, Scranton R, Pepin J, Ramamoorthy S. Impact of postsurgical opioid use and ileus on economic outcomes in gastrointestinal surgeries. Curr Med Res Opin. 2015;31(4):677-686. doi:10.1185/03007995.2015.1005833
8. Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071. doi:10.1001/jamasurg.2017.0831
9. Understanding the epidemic. Centers for Disease Control and Prevention. Last reviewed March 17, 2021. Accessed June 16, 2022. https://www.cdc.gov/drugoverdose/epidemic
10. FDA considers new approach to improve safe disposal of prescription opioid analgesics, decrease unnecessary exposure to unused medication. News Release. FDA. April 20, 2022. Accessed August 15, 2022. https://www.fda.gov/news-events/press-announcements/fda-considers-new-approach-improve-safe-disposal-prescription-opioid-analgesics-decrease-unnecessary
11. EXPAREL®. Prescribing information. Pacira Pharmaceuticals, Inc; 2022. Accessed June 23, 2022. https://www.exparel.com/hcp/prescribing-information.pdf
12. King PM, Blazeby JM, Ewings P, et al. The influence of an enhanced recovery programme on clinical outcomes, costs and quality of life after surgery for colorectal cancer. Colorectal Dis. 2006;8(6):506-513. doi:10.1111/j.1463-1318.2006.00963.x
13. Thiele RH, Rea KM, Turrentine FE, et al. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg. 2015;220(4):430-443. doi:10.1016/j.jamcollsurg.2014.12.042
14. Miller TE, Thacker JK, White WD, et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg. 2014;118(5):1052-1061. doi:10.1213/ANE.0000000000000206
15. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536. doi:10.1016/j.knee.2011.12.004
16. Richard BM, Newton P, Ott LR, et al. The safety of EXPAREL® (bupivacaine liposome injectable suspension) administered by peripheral nerve block in rabbits and dogs. J Drug Deliv. 2012;2012:962101. doi:10.1155/2012/962101
17. Patel MA, Gadsden JC, Nedeljkovic SS, et al. Brachial plexus block with liposomal bupivacaine for shoulder surgery improves analgesia and reduces opioid consumption: results from a multicenter, randomized, double-blind, controlled trial. Pain Med. 2020;21(2):387-400. doi:10.1093/pm/pnz103
18. Niraj G, Kelkar A, Powell R. Ultrasound-guided subcostal transversus abdominis plane block. Int J Ultrasound Appl Technol Perioper Care. 2010;1(1):9-12. doi:10.5005/jp-journals-10014-1002
19. Børglum J, Jensen K. Abdominal surgery: advances in the use of ultrasound-guided truncal blocks for perioperative pain management. In: Derbel F, ed. Abdominal Surgery. InTech; 2012:69-94.
20. Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621-627. doi:10.1097/AAP.0000000000000451
21. Go R, Huang YY, Weyker PD, Webb CA. Truncal blocks for perioperative pain management: a review of the literature and evolving techniques. Pain Manag. 2016;6(5):455-468. doi:10.2217/pmt-2015-0012
22. Keller DS, Pedraza R, Tahilramani RN, Flores-Gonzalez JR, Ibarra S, Haas E. Impact of long-acting local anesthesia on clinical and financial outcomes in laparoscopic colorectal surgery. Am J Surg. 2017;214(1):53-58. doi:10.1016/j.amjsurg.2015.10.035
23. Keller DS, Tahilramani RN, Flores-Gonzalez JR, Ibarra S, Haas EM. Pilot study of a novel pain management strategy: evaluating the impact on patient outcomes. Surg Endosc. 2016;30(6):2192-2198. doi:10.1007/s00464-015-4459-4
24. Beck DE, Margolin DA, Babin SF, Russo CT. Benefits of a multimodal regimen for postsurgical pain management in colorectal surgery. Ochsner J. 2015;15(4):408-412.