Article
Three Studies in CV Risk Management: What's the Practice Impact?
Author(s):
Oral anticoagulant choice in the obese; how to reduce inflammation in atherosclerosis; and optimal DAPT duration post-PCI. Top-line reviews of 3 new studies highlight potential for practice change.
Three studies published in the first half of 2019 are notable for their contributions to challenging areas of treatment in cardiovascular medicine -- stroke management in obese patients; reduction of systemic inflammation in patients with, or at risk for, atherosclerosis; and, how to choose the optimal duration of dual-antiplatelet therapy after stenting and revascularization for complex patients.
Following are concise reviews of studies originally published in the American Heart Journal,New England Journal of Medicine and JACC.
Rivaroxaban vs warfarin

The risk for stroke and bleeding appears to be similar when morbidly obese patients with atrial fibrillation (AF) are treated with warfarin vs rivaroxaban, according to a study recently published in the American Heart Journal.
The study also found that annual costs per patient per year (PPPY) were significantly lower with rivaroxaban vs warfarin.
“Our data suggest that morbidly obese patients with AF who were matched for comorbidities and underlying risks can achieve similarly effective anticoagulation with rivaroxaban as with warfarin,” wrote researchers led by Eric Peterson, MD, MPH, of Duke University School of Medicine in Durham, North Carolina.
People with morbid obesity are at increased risk for AF. While anticoagulation has long been the standard of care for AF, obese patients may have different drug metabolism and different dosing needs. Studies of obese patients on warfarin have shown that these patients have lower initial response and take longer to reach therapeutic levels vs non-obese individuals.
Rivaroxaban is an agent in the new class of direct-acting oral anticoagulants (DOACs). Studies have suggested that rivaroxaban may not require dose adjustment in obese patients. However, the safety and efficacy of DOACs in morbidly obese patients has not been established. For this reason, the International Society of Thrombosis and Hemostasis has recommended against using DOACs in morbidly obese patients.
To fill in the knowledge gaps about rivaroxaban in morbid obesity, researchers analyzed real-world data from US commercial insurance and Medicare claims databases from 2010 to 2016.
No significant difference in risk.

The study included 3563 matched pairs of people with AF and morbid obesity (body mass index >40 kg/m2 or weight >265 lbs). Individuals on rivaroxaban were matched to those on warfarin by underlying factors that may contribute to stroke and bleeding.
Over an average of 10 months, risk for stroke/embolism and major bleeding did not differ significantly between the warfarin and rivaroxaban groups.
However, total annual healthcare costs PPPY were significantly lower with rivaroxaban ($48 552) vs warfarin ($52 418), a $3890 difference.
The main reason for lower costs with rivaroxaban were significantly lower rates of hospitalization, shorter hospital stays, and fewer outpatient visits.
Rivaroxaban generally does not need routine monitoring for anticoagulation, which may explain the difference in outpatient visits. Patients on warfarin require frequent monitoring-nearly monthly–to ensure that their anticoagulation is in the therapeutic range.
No reduction in CV risk with methotrexate.

Low-dose methotrexate vs placebo does not appear to decrease cardiovascular (CV) risk in people with atherosclerosis, according to results from the Cardiovascular Inflammation Reduction Trial (CIRT) published recently in the New England Journal of Medicine.1
Inflammation likely plays a key role in the development of atherosclerosis, yet this hypothesis remains to be proven. The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) lent support to this idea when it showed that the anti-inflammatory drug canakinumab was tied to decreased CV events.2
Methotrexate represents a less expensive anti-inflammatory option. The drug is widely used to treat autoimmune conditions like rheumatoid arthritis. Studies have found that patients with these conditions have fewer CV events when treated with methotrexate, but the drug’s impact on CV risk remains uncertain.
To further evaluate the issue, researchers conducted a randomized, double blind trial. The study included 4786 patients with high CV risk and a history of heart attack or multivessel coronary artery disease in addition to type 2 diabetes or metabolic syndrome. Participants were randomized to low-dose methotrexate or placebo for 8 months. Participants were 19% women, and 22% nonwhite/Hispanic.
The trial was stopped early after 2.3 years when it became clear that methotrexate was not associated with decreased CV events.
Methotrexate has no impact on inflammatory biomarkers.

Results showed that methotrexate was not linked to lower markers of inflammation, including interleukin-1β, interleukin-6, or C-reactive protein, vs placebo.
Risk of the primary endpoint, a composite of major CV events (nonfatal myocardial infarction, nonfatal stroke, or CV death) was not significantly different between low-dose methotrexate and placebo. There was also no significant difference in CV death: 49 patients died from CV causes in the methotrexate group vs 43 with placebo.
Methotrexate was linked to elevations in liver enzymes, decreased white blood cell count, decreased hematocrit and hemoglobin levels, and increased non-basal cell skin cancer.
The particular immune pathway targeted by a drug may play a role in CV risk, the authors noted. Canakimuab targets the interleukin-1β–interleukin-6 pathway, which may be more important in the development of atherosclerosis than other immune pathways. This difference may explain why only canakimab has been tied to decreased CV events, while other anti-inflammatories like methotrexate have failed to show an association.
“The observations in CANTOS, CIRT, and other trials highlight the importance of considering the mechanistic diversity of inflammatory pathways in atherosclerosis and of exploring approaches to their inhibition,” wrote researchers led by Paul Ridker, MD, of Brigham and Women’s Hospital in Boston, Massachusetts.
Short-term DAPT best in complex PCI.
Patients who undergo complex PCI and do not have a high bleeding risk (HBR) are the most likely to benefit from long-term DAPT. Results from the PRECISE-DAPT study (JACC, 2019) suggest that bleeding risk should outweigh risk for ischemic events when deciding about long-term DAPT in this group.
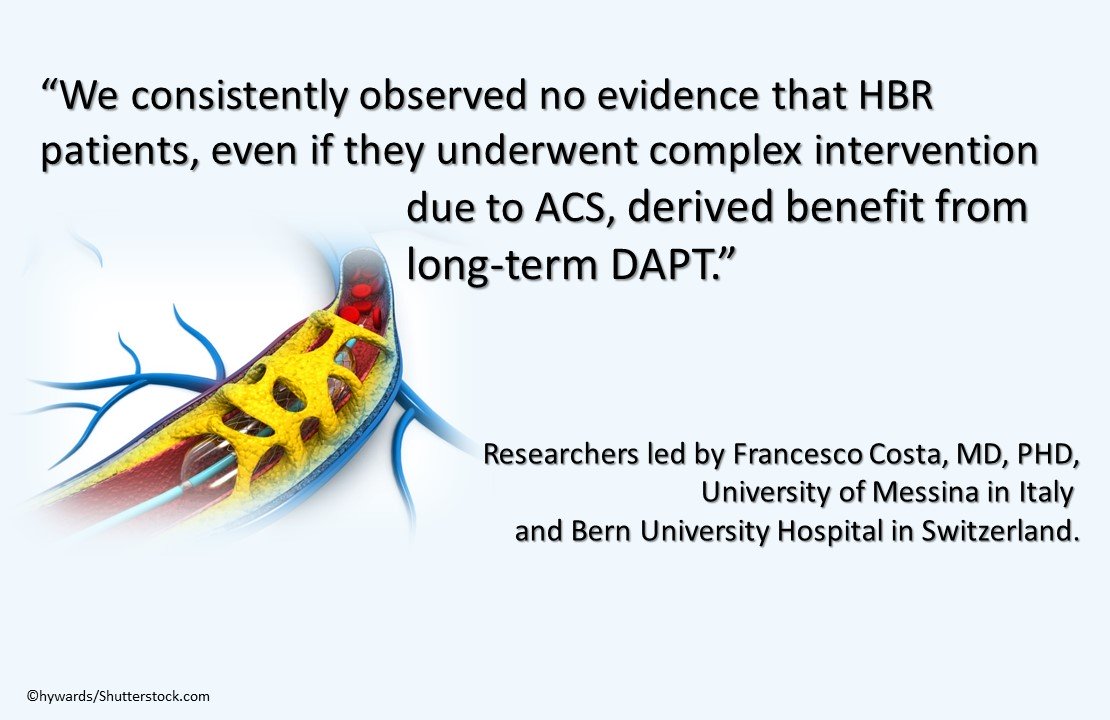
In patients who receive complex PCI and have a HBR, long-term DAPT does not provide any additional reduction in risk for ischemic events and may increase risk for bleeding. Such patients may benefit most from short-term DAPT.
“We consistently observed no evidence that HBR patients, even if they underwent complex intervention due to ACS, derived benefit from long-term DAPT,” wrote researchers led by Francesco Costa, MD, PhD, of the University of Messina in Italy, and Bern University Hospital in Switzerland.
Patients who undergo complex PCI-defined in this study as stenting of ≥3 lesions, bifurcation stenting, stent length >60 mm,
and/or chronic total occlusion revascularization – have increased risk for ischemic events.
Some studies suggest that long-term DAPT can decrease ischemic risk in such patients better vs short-term DAPT. However, such patients often have multiple medical problems, which can increase their risk for bleeding. So, it is not always clear how to balance ischemic vs bleeding risk.
Complex PCI in 3000 patients.
To help clinicians decide which patients may benefit most from long-term DAPT, researchers pooled data from 8 RCTs. The analysis included almost 3000 patients who had undergone complex PCI.
In most studies, patients were assigned to short-term DAPT (3 or 6 months) or long-term DAPT (12 or 24 months).
To estimate bleeding risk, the study used the 5-item PRECISE-DAPT score. Ischemic risk was estimated using guideline-endorsed criteria.
Results showed that long-term DAPT was linked to a 3.5% decreased risk of ischemic events in complex PCI patients without HBR, with a negligible effect on bleeding risk vs short-term DAPT.
Complex PCI patients with HBR showed no net clinical benefit from long-term DAPT. This group had a 1% increase in ischemic events and almost 2% increase in bleeding risk with long-term vs short-term DAPT.
The authors mentioned the study is hypothesis-generating only. Further studies are needed to confirm results.
-------------------------------------------------------
Veronica Hackethal, MD, is a contributing author and freelance medical writer living in New York City.





