Article
Upadacitinib Effective on for Atopic Dermatitis Patients with Concomitant Hand Eczema
Author(s):
New research on updadacitinib’s use for atopic dermatitis patients suggests the drug may be effective for hand eczema.
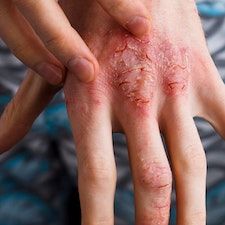
Upadacitinib was found to be effective following 16 weeks of treatment for atopic dermatitis (AD) patients with concomitant hand eczema, according to recent findings.
This study’s investigators set out to assess both the effectiveness and safety of upadacitinib treatment for AD and on HE in AD patients, using the multi-center Dutch BioDay registry.
Recent developments for AD patients had shown promise but some also showed promise for HE, according to the study team.
The researchers for this study were led by Esmé Kamphuis, from the University Medical Center Groningen’s Department of Dermatology at University of Groningen in the Netherlands.
“Only three clinical daily practice studies and a few case series/reports have been published,” Kamphuis and colleagues wrote. “Moreover, daily practice data of the effectiveness of upadacitinib on HE in AD patients has not been published. Therefore, the aim of this prospective BioDay registry study is to evaluate the 16-week effectiveness and safety of upadacitinib on AD and HE in AD patients in daily practice.”
Background
The study was a multicenter, prospective, observational, cohort study which used data from the BioDay registry in the Netherlands.
BioDay was noted for including patients from both the University Medical Center Groningen and the Medical Center Leeuwarden dermatology departments, and the registry’s data centers on AD patients who are being treated with biologics or small molecules.
The investigators included all AD patients in the registry who had been treated with upadacitinib from September of 2021 and June of 2022.
The study participants had been given 15 mg once daily at baseline, although those with severe clinical presentation and a notable history of disease were treated with 30 mg.
Study Findings
The research team ended up with 32 patients with HE, out of 38 total patients, and they used Eczema Area and Severity Index (EASI) 25 as well as the Investigator's Global Assessment (IGA) rating systems to assess them.
The team defined the outcomes’ endpoints—reported by clinicians—as the proportion of study participants who reported EASI scores of either ≥50%, ≥75% or ≥90% compared to baseline.
The investigators ended up finding that EASI-75 was achieved by exactly 50% of participants, demonstrating solid results for the treatment.
In assessing their other outcomes, the team noted that 62.5% of patients reported an absolute cutoff score of ≤4 on the Numeric Rating Scale (NRS), 37.5% reported a Patient-Oriented Eczema Measure (POEM) of ≤7, and 59.4% reported a ≤5 score on the Dermatology Life Quality Index (DLQI).
“Upadacitinib can be an effective treatment for patients with AD and concomitant HE in daily practice,” they wrote. “Future studies should focus on the effectiveness of upadacitinib on chronic HE, especially on the different etiological subtypes of HE, including HE in non-atopic individuals.”
The study, “Experiences from daily practice of upadacitinib treatment on atopic dermatitis with a focus on hand eczema: Results from the BioDay registry,” was published online in Contact Dermatitis.





