Article
Using DOACs in People with Chronic Kidney Disease, with Kim Zuber, PA-C, and Jane Davis, DNP
Author(s):
Kim Zuber, PA-C, and Jane Davis, DNP, offer insight into the use of DOACs in people with atrial fibrillation and chronic kidney disease.
Jane Davis, DNP (left) and Kim Zuber, PA-C (right)
Credit: Self

Atrial fibrillation (AF) is linked to an increase in embolic strokes and thus most patients with AF will be prescribed anticoagulants to decrease this incidence.
AF is frequent in chronic kidney disease (CKD) with estimates ranging from 16-21% in non-dialysis patient and up to 40% in dialysis patients.1 AF is associated with both worse cardiac and kidney outcomes with an increased risk of CKD progression.1 The incidence of stroke in End Stage Kidney Disease (ESRD) is 7-9 times greater than in the general population and 3-fold more deadly.2
For many years, warfarin was the drug of choice to decrease strokes in all populations but serum monitoring requirements, diet restrictions and narrow therapeutic window led to multiple complications. With the introduction of the direct oral anticoagulant (DOACs) medications, the complication rate in AF was significantly decreased.
As is true for most drug trials, patients with severe CKD (defined as an eGFR <25 ml/min) were excluded from the DOAC trials.3 This is not unexpected; kidney patients have multiple comorbidities and are treated with multiple medications.
These comorbidities and medications add a level of complexity to the evaluation of adverse events during a drug trial. Yet, for a population where close to half of the population has AF, we do not have answers about the use of DOACs.
As there is no guidance from the pivotal DOAC trials for the CKD and ESRD patients, the kidney community has undertaken the collection of data from real world use of DOACs. We know that acute kidney injury (AKI) is significantly lower with the use of DOACs vs warfarin.4 This is true for all stages of CKD, even in the elderly.
Using a meta-analysis approach, we have seen that DOACs have a better safety and effectiveness profile than warfarin.5 For this reason, the Kidney Disease Improving Global Outcomes (KDIGO) expert workgroup has developed recommendations for use of DOACs in CKD.1
Adapted from KDIGO Controversies Conference1
Legend: KDIGO-Kidney Disease Improving Global Outcomes, SCr-serum creatinine, eGFR-estimated glomerular filtration rate, bid-twice a day, qd-once a day.
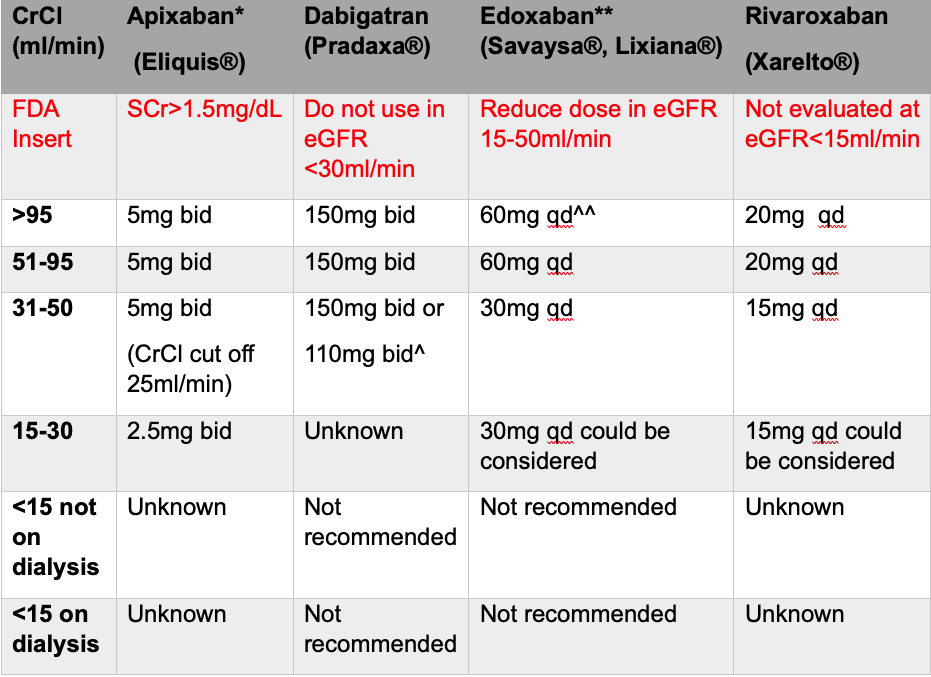
Yet, there is still a population that KDIGO recommendations did not address: the ESRD patient.
In the kidney failure patient, the incidence of AF is 40% and the stroke and sudden cardiac death are common.1 While cardiology defers to nephrology when treating ESRD, nephrology is divided on optimal treatment for this population. Most trials are retrospective and observational. However, recently randomized, controlled trials have been conducted.
The trial arms include:
- A DOAC (apixaban is the most studied)
- Warfarin
With the most recent trial data available, the consensus is that warfarin is the most dangerous for this population whether it is used for AF prophylaxis or treatment for recurrent thromboembolism.6,7 Warfarin increases the incidence of bleeding without decreasing mortality. Two other large trials looking at DOAC vs warfarin both showed a better safety profile for apixaban but there were significant episodes of bleeding in the dialysis population.8,9 For this reason, the recently closed preliminary trial of three separate trial arms, which examine apixaban, warfarin, and no treatment, respectively, is being closely watched.10
This Canadian/Australian trial included 151 participants and is a pilot to evaluate if we should NOT be treating the dialysis population with anticoagulants. The trial was completed in late December 2022. Results are pending.
If, as expected, the no treatment arm has a similar outcome profile with less bleeding complications, we will have to reassess our mantra of ‘First, do no harm’ while we stop treating the AF patient on dialysis with anticoagulants. A definite relearn for all of us.
References:
- Wanner C, Herzog CA, Turakhia MP; Conference Steering Committee. Chronic kidney disease and arrhythmias: highlights from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2018 Aug;94(2):231-234.
- Bobot M, Suissa L, Hak JF, et al. Kidney disease and stroke: epidemiology and potential mechanisms of susceptibility. Nephrol Dial Transplant. 2023 Feb 8:gfad029.
- Garlo KG, Steele DJR, Nigwekar SU, et al. Demystifying the Benefits and Harms of Anticoagulation for Atrial Fibrillation in Chronic Kidney Disease. Clin J Am Soc Nephrol. 2019 Jan 7;14(1):125-136.
- Harel Z, McArthur E, Jeyakumar N, et al. The Risk of Acute Kidney Injury with Oral Anticoagulants in Elderly Adults with Atrial Fibrillation. Clin J Am Soc Nephrol. 2021 Oct;16(10):1470-1479.
- Malhotra K, Ishfaq MF, Goyal N, et al. Oral anticoagulation in patients with chronic kidney disease: A systematic review and meta-analysis. Neurology. 2019 May 21;92(21):e2421-e2431
- Wetmore JB, Herzog CA, Yan H, et al. Apixaban versus Warfarin for Treatment of Venous Thromboembolism in Patients Receiving Long-Term Dialysis. Clin J Am Soc Nephrol. 2022 May;17(5):693-702.
- Mavrakanas TA. Should Oral Anticoagulation Be Used in ESKD Patients on Hemodialysis with Atrial Fibrillation?: COMMENTARY. Kidney360. 2021 Apr 9;2(9):1412-1414.
- Pokorney SD, Chertow GM, Al-Khalidi HR, et al for the RENAL-AF Investigators. Apixaban for Patients With Atrial Fibrillation on Hemodialysis: A Multicenter Randomized Controlled Trial. Circulation. 2022 Dec 6;146(23):1735-1745.
- Reinecke H, Engelbertz C, Bauersachs R, et al. A Randomized Controlled Trial Comparing Apixaban With the Vitamin K Antagonist Phenprocoumon in Patients on Chronic Hemodialysis: The AXADIA-AFNET 8 Study. Circulation. 2023 Jan 24;147(4):296-309.
- Strategies for the management of atrial fibrillation in patients receiving dialysis (Safe-D) ClinicalTrials.gov identifier NCT03987711





