Article
Ustekinumab Quickly, Effectively Treats Patients with Active PsA
Author(s):
In the prospective, non-interventional SUSTAIN study, patients with active psoriatic arthritis received ustekinumab for 3 years in routine clinical care.
Patients with active psoriatic arthritis (PsA) who were treated with ustekinumab for 3 years (160 weeks) reported rapid onset of effect, which was maintained throughout treatment. The drug was also highly tolerated within this patient population, according to a study published in Springer.1
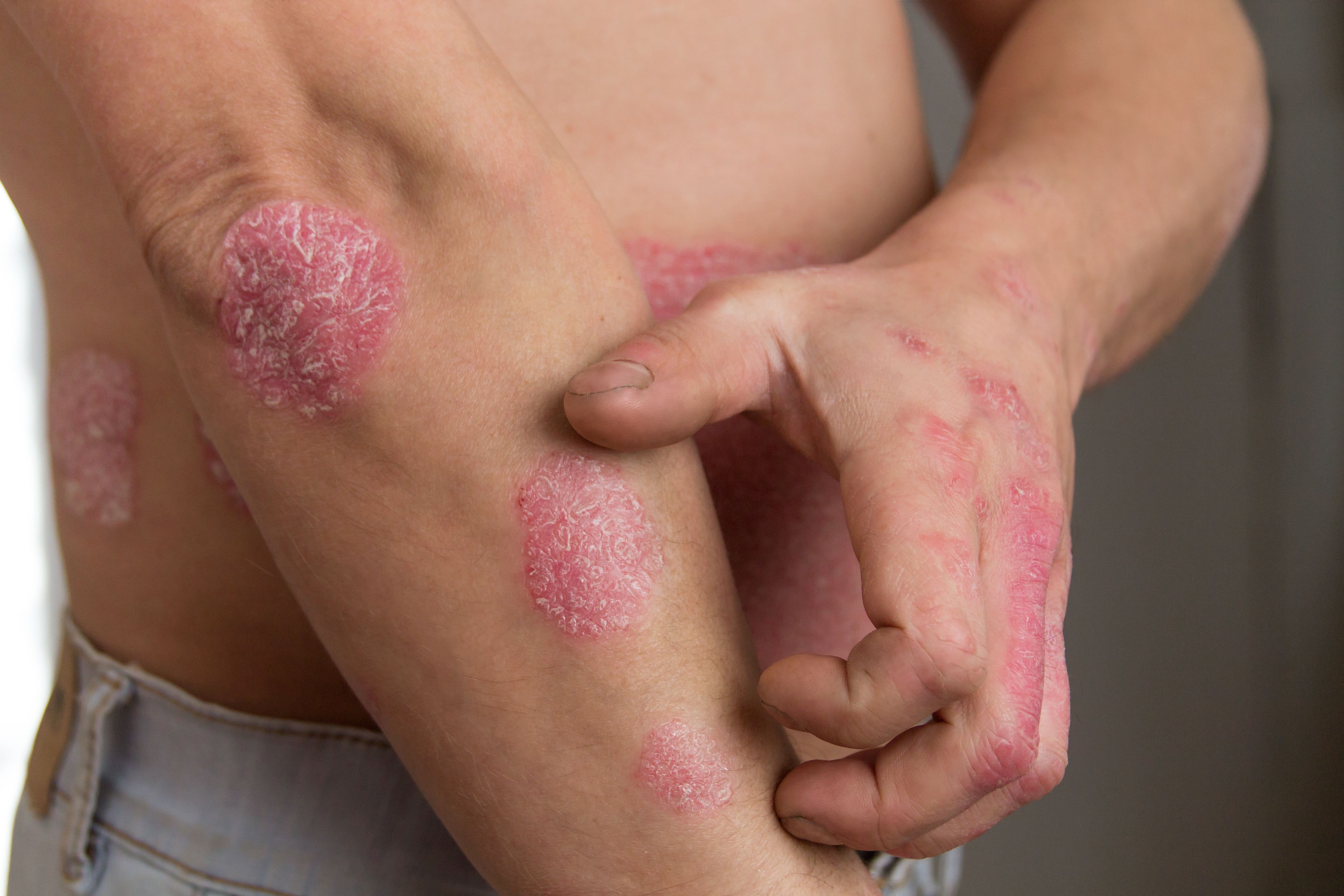
“The primary aim of PsA treatment is to provide prolonged treatment effects with minimal joint damage due to disease progression, and to improve patient quality of life (QoL),” investigators explained. “Current recommendations support a treat-to-target approach, where patients are closely monitored and treatment is modified with the goal of reaching remission or low disease activity, while also considering the different disease domains involved.”
In the prospective, non-interventional SUSTAIN study, conducted at 74 sites in Germany, patients with active PsA received ustekinumab for 3 years in routine clinical care. Assessments at baseline, week 4, and every 12 weeks thereafter were collected and evaluated. Eligible patients were aged 18 years or older, had a confirmed diagnosis of active PsA, and a previous inadequate response to a disease-modifying antirheumatic drug (DMARD).
Of the original 337 patients initially enrolled in the analysis, 129 (38.3%) continued treatment until week 160. Nearly all (n = 123, 95.3%) of patients had received previous PsA medication, which included biologics. At baseline, 47.3% of patients had peripheral large joints affected by PsA, 72.9% had small joints affected by the condition, and 14 patients (10.9%) had axial involvement. There was a decrease from baseline to week 4 for tender joint count (TJC) (8.0 to 5.8) and swollen joint count (SJC) (4.5 to 3.1). Decreases continued to week 28 and sustained to week 160 (1.0 and 0.4, respectively). Skin improvements in patients with PsA and psoriasis (PsO) were seen at week 4, continued to week 28, and were maintained until week 160. Additionally, patient-assessed pain, sleep quality, and health scores showed similar patterns of response.
Regardless of the number of prior biologic therapies, improvements were shown in TJC, SJC, affected body surface area, and Psoriasis Area and Severity Index (PASI). Minimal disease activity was achieved in 31.9% (n = 36) patients by week 28 and 33.6% (n = 38) at week 52.
Adverse events (AEs) related to ustekinumab were reported in 47.3% (n = 61) of patients and serious AEs were reported in 3.1% (n = 4) of patients. At the end of the study, 100% of patients assessed the tolerability of the drug as either good or very good.
The lack of a comparator group limits the SUSTAIN study and results may not be generalizable to patients in other countries as it is a German-based study. Additionally, only patients who continued treatment with ustekinumab for the entirety of the 3-year period were included in the analysis, which might indicate that efficacy and safety are limited to those who benefited from the treatment. Therefore, the effectiveness of the drug may be overestimated. Lastly, parameters may have been reported infrequently and inconsistently, as clinicians documented comorbidities according to personal preference.
“This analysis of the SUSTAIN study demonstrates that, in routine clinical practice, ustekinumab can provide rapid and sustained (long-term) treatment efficacy for up to 160 weeks (3 years), as well as high tolerability, in patients with active PsA who respond to ustekinumab treatment,” investigators concluded. “As the primary aim of any physician treating PsA is to prescribe a well-tolerated therapy, with fast onset and sustained efficacy that also improves QoL, we propose that ustekinumab offers a valuable therapeutic option.”
Reference:
Wendler J, Damann N, Röcken M, et al. Ustekinumab Is Rapid-Acting and Is an Effective Long-Term Treatment for Patients with Active Psoriatic Arthritis: Real-World Evidence from the Non-interventional SUSTAIN Study [published online ahead of print, 2022 Sep 6]. Rheumatol Ther. 2022;10.1007/s40744-022-00484-3. doi:10.1007/s40744-022-00484-3




