Article
Vildagliptin and Linagliptin Associated with Bullous Pemphigoid in Japan
Author(s):
Dipeptidyl peptidase-4 inhibitors have been linked to cases of bullous pemphigoid across Japan, prompting investigators to suggest trying DPP-4i discontinuation followed by supportive care.
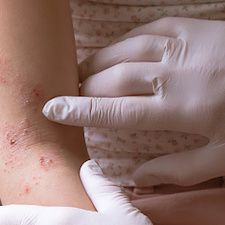
New data into dipeptidyl peptidase-4 inhibitor (DPP-4i)-associated cases of bullous pemphigoid confirmed that vildagliptin and linagliptin were the DPP-4i commonly associated with the rare skin condition.
Dipeptidyl peptidase-4 inhibitors have been widely used for the management of type 2 diabetes mellitus, and consequently have resulted in cases of BP across Japan. Despite the incidence rate, no large-scale surveys have been conducted in the country.
As such, an investigative team led by Seiko Sugiyama, MD, from the Kawaski Medical School General Medical Center, conducted a nationwide study with the intention of determining the characteristics and clinical course of DPP-4i-associated BP in Japan.
Data from 94 facilities across the country were utilized in the study following a secondary questionnaire issued to 669 dermatology departments and clinics in certified dermatologist training facilities in the Japan Dermatological Association.
The data pertained to incident cases diagnosed as BP from January 1 to December 31, 2016, which were collected via postal questionnaire surveys. Additional data included DPP-4i use or not at the time of BP diagnosis, the type of DPP-4i, age at diagnosis, sex, Bullous Pemphigoid Disease Area Index (BPDAI), treatment details and more.
Patients treated with a DPP-4i who developed BP were featured in the DPP-4i group while those not taking a DPP-4i who developed BP were featured in the controls group.
During the aforementioned timeframe, a total of 713 new BP patients were reported in 2016, with a mean of 7.59 patients per facility. Among them, 243 (34.1%) were taking DPP-4i while 461 (64.7%) were not. Data were missing for 9 patients included in the study.
The most common gliptins administered to patients with DPP-4i-BP were vildagliptin (37.2%) and linagliptin (23.8%).
The mean age of BP did not differ significantly among those treated with DPP-4i and controls, nor did the severity of BP. However, non-inflammatory BP was shown to be more common among those in the treatment group (P<0.01), and the BP230 antibody titer was significantly higher in the control group (P<0.01).
Notably, DPP-4i therapy was discontinued after BP diagnosis in 79.9% of patients, and 17.6% achieved remission with discontinuation alone or in conjunction with supportive care.
Despite DPP-4i-associated BP not having a specific clinical type or serum profile, investigators found that those treated with DPP-4i who developed BP were at a higher risk of impaired glucose tolerance from steroids compared to the general BP population in Japan.
“We suggest trying DPP-4i discontinuation followed by supportive care before resorting to systemic corticosteroids and/or adjuvant therapy in these patients,” investigators concluded.
The study, "Clinical features of dipeptidyl peptidase-4 inhibitor-associated bullous pemphigoid in Japan: A nationwide retrospective observational study," was published online in the Journal of Dermatology.





