Article
What role do occupational exposures play in RA?
Occupational exposure to various chemicals, minerals, and toxins may increase the risk of rheumatoid arthritis (RA). Relationships between silica exposure and lung, renal, and autoimmune disease have been observed. Although a relationship between silica exposure and RA has been identified, it is not well defined. The evidence indicating that cigarette smoking is an independent risk factor for RA is conclusive. Agents that may be capable of inducing experimental arthritis in animals include adjuvants from bacteria, yeast, viruses, and mineral oils. In a Swedish study, exposure to any mineral oil was associated with a 30% increased relative risk of RA. (J Musculoskel Med. 2008;25:130-136)
ABSTRACT: Occupational exposure to various chemicals, minerals, and toxins may increase the risk of rheumatoid arthritis (RA). Relationships between silica exposure and lung, renal, and autoimmune disease have been observed. Although a relationship between silica exposure and RA has been identified, it is not well defined. The evidence indicating that cigarette smoking is an independent risk factor for RA is conclusive. Agents that may be capable of inducing experimental arthritis in animals include adjuvants from bacteria, yeast, viruses, and mineral oils. In a Swedish study, exposure to any mineral oil was associated with a 30% increased relative risk of RA. (J Musculoskel Med. 2008;25:130-136)
Autoimmunity, defined as the development of an immune system response in the form of autoantibodies and T-cell responses to self-structures, has been researched widely over the years because it is prevalent in men and women from childhood throughout their adult years. Patterns of disease occurrence in families and in animal models strongly suggest a role for genetic factors. The genes most frequently associated with autoimmunity are those that regulate immune responses and cytokine production.
Environmental factors also have been hypothesized to play a role in the development of autoimmune diseases. Studies have provided strong support for the concept that these diseases result from interactions of both genetic and environmental factors.
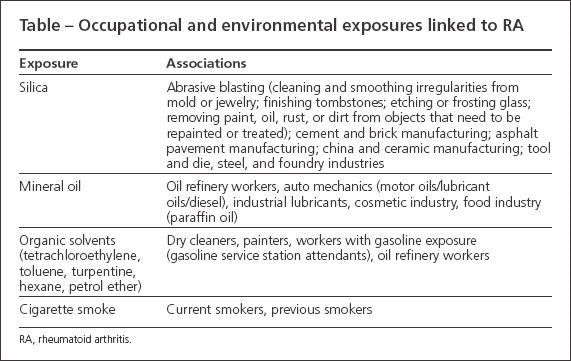
A growing amount of the medical literature now claims that the development of rheumatoid arthritis (RA) may be influenced by occupational exposure to various chemicals, minerals, and toxins (Table). Studies that examined the role of silica and mineral oil exposure in RA have found significant associations and increased risk. In this article,we review the existing epidemiological and experimental literature on occupational exposures and their correlation to the development of RA.
METHODS AND RESULTS
Cross-referencing the terms "occupational exposure" and "environmental exposure" with the term "rheumatoid arthritis," we searched MEDLINE (1966 - 2006) and PubMed (1966 - 2006) for articles in English that were human studies. Retrieved articles were selected based on their clinical relevance and applicability.
Silica exposure and increased health risk
Epidemiological evidence has stimulated investigation into occupational exposure to suspected pathogens and carcinogens and any resultant increased health risk.The relationship between occupational silica exposure and lung disease, primarily in occupations associated with mining, has a well-documented history; accounts of occupational exposure have accumulated much evidence indicating a direct increased health risk, specifically for lung pathology.1-3 More in-depth studies are related to exposure limits, occupations with increased risk, and additional health risks related to silica exposure.4-6
Relationships between silica exposure and renal disease and autoimmune disease also have been observed.2,7-11 A relationship between silica exposure and RA has been identified, although the relationship currently is not well defined.12
Coexisting pulmonary and rheumatologic findings
In the early 1950s, Caplan13 was one of the first to document the coexistence of pulmonary and rheumatologic findings, showing chest radiograph opacities in coal miners with known RA. Further studies investigated and confirmed the positive correlation between RA and silicosis and noted that lung fibrosis is more likely to develop in workers exposed to silica who have RA than others who have similar exposure.14
Information from studies of Welsh and South African coal miners suggested an autoimmune cause for the development of more severe, symptomatic silicosis in workers who have silica exposure.14,15 Workers who had abnormal chest radiograph results-but who did not have RA-had high rates of positive serological findings, including an increase in rheumatoid factor (RF) in coal miners16 and of antinuclear antibodies in sandblasters.17
These results are far from conclusive given the heterogeneity of the studies and the lack of adjustment for confounding factors, such as cigarette smoking. However, the studies raised sufficient questions to warrant further study of the role of silica exposure in the onset and pathogenesis of RA.
Increased risk in coal mining
Turner and Cherry18 investigated the relationship between occupational exposure to silica in pottery manufacturing and RA in a retrospective review of men and women employed in the pottery, refractory metal, and sandstone industries. Variables included exposure, cigarette smoking as a confounding variable, employment in the coal-mining industry, and number of pregnancies.They did not find a relationship between mean concentration of exposure to silica and RA, and there was no clear evidence of increased risk of RA attributable to silica exposure.There was an increased risk identified for men who had worked in coal mining.
Postmortem retrospective
Calvert and associates19 further investigated the suggestion that silica is a risk factor for RA. In their postmortem retrospective analysis, death certificate data from 27 states were collected.
Significant increases in mortality odds ratio were observed for RA, in addition to silicosis, chronic obstructive pulmonary disease, and pulmonary tuberculosis. The mortality odds ratios for silica exposure categories were 1.23, 1.11, 1.05, and 1.19 for “medium exposure,” “high exposure,” “super high exposure,” and “ever been exposed” (all categories combined) versus “low/no exposure,” respectively. Although the results indicate a significant risk of RA associated with any silica exposure, this relationship may not be linearly related to quantity of silica exposure.
The results also reveal an important consideration: there may not be a clear threshold limit for exposure. This emphasizes the importance of determining the attributable risk for exposure and the safety precautions needed to limit exposure.
Silica exposure an independent risk factor?
The Swedish Epidemiological Investigation of Rheumatoid Arthritis (EIRA), a large population-based, case-control study that used incident cases of RA, sought to control for cigarette smoking and examine silica exposure as an independent risk factor.20 Of 276 men, 41 were designated as silica-exposed (by self-report of having worked with rock drilling or stone crushing or previous exposure to stone dust). Silica-exposed men had an increased risk of RA (odds ratio 2.2 in men aged 18 to 70 years and 2.7 in those aged 50 to 70 years). Men who had worked in rock drilling or stone crushing had a slightly greater increase in risk of RA than silica-exposed men. There was no significant difference between RF-positive and RF-negative odds ratios. Silica exposure was found to be an independent risk factor for RA; however, cigarette smoking further increased the apparent risk.
Other epidemiological studies also have investigated the association between exposure to silica through the respiratory tract and the risk of RA.16,21 In these studies, an increased risk of RA was observed in silica-exposed persons in some occupations and workplaces and with silicosis. However, the number of observed cases was small in 3 of the studies and only 2 of them defined cases according to established clinical criteria.
Relationship not well defined
Although these data reinforce the existence of a relationship between silica exposure and RA, the terms of this relationship still are not well defined. Silica exposure appears to be an independent variable related to the development of RA, but defining silica exposure is not objective. Given the available cumulative evidence relating various disease states to industries with high rates of occupational silica exposure, efforts to limit silica exposure certainly should be endorsed.
The evidence indicating that cigarette smoking is an independent risk factor for RA is conclusive,22 with an increased risk in seropositive subjects versus seronegative subjects.23-25 However, more research is needed to identify the scope of the genetic susceptibilities, further define their implications with respect to environmental exposures, and identify interventions that may influence disease development and progression. Additional work also is needed to define objective quantification of silica exposure limits and promotion of allowable safe work standards.
Although the exact mechanisms behind the biological effects of silica are not fully understood, inflammatory mediator activation during phagocytosis is thought to play a role.26,27 The surface properties of silica may be responsible for cellular activation and resultant production of chemokine and inflammatory mediators.28
Macrophages may trigger systemic activation of additional macrophages and monocytes. These cell types also can increase the production of matrix metalloproteinases (MMPs), oxygen-derived free radicals (ODFRs), nitric oxide, and fibrogenic cytokines. ODFRs and nitric oxide have been the subject of investigation because evidence has accumulated about their role as chemical mediators and resulting cellular damage. 29,30 Activation of MMPs is of particular interest because these enzymes are involved in extracellular matrix degradation and remodeling, which have been shown to be associated with RA.31
Adjuvants from many sources
Silica is not the only agent that may have a pathophysiological relationship to RA. Those capable of inducing experimental arthritis in animals, particularly in rodents, include adjuvants that originate from many sources, such as bacteria, yeast, viruses, and mineral oils.32-34 In several animal models, arthritis develops in rodents with some genetical backgrounds after they are exposed to nonimmunogenical adjuvants, both intradermally and percutaneously.35-36
Research investigating the exact mechanisms that are involved in the pathogenesis of these adjuvant arthritis models continues.37-38 Current areas of investigation include recruitment of CD4+ and CD8+ T cells, tumor necrosis factor α, cellular adhesion molecules, cytokine elaboration, and major histocompatibility complex determinants. 37-40 It is well established that mineral oil is a nonimmunogenic adjuvant that can induce an autoimmune response, specifically arthritis, in genetically susceptible rats.35,36,41 This basic science research provides the background evidence for investigation of adjuvant-induced arthritis in humans.
Risk with organic solvents
Lundberg and colleagues42 provided one of the first pieces of epidemiological evidence of an increased relative risk of RA with exposure to organic solvents. In another epidemiological survey, Olsson and coworkers43 also found an increased risk of RA in men employed at service stations.
The EIRA study in Sweden furthered investigation into the relationship between mineral oil and arthritis in humans.20 Antibodies for RF and anticyclic citrullinated peptide (anti-CCP) were assayed, in addition to DNA genotype for the presence of shared epitope genes.
References:
References
- 1. Hnizdo E, Sluis-Cremer GK, Abramowitz JA. Emphysema type in relation to silica dust exposure in South African gold miners [published correction appears in Am Rev Respir Dis. 1991;144:1223]. Am Rev Respir Dis. 1991;143:1241-1247.
- 2. Berry G, Rogers A, Yeung P. Silicosis and lung cancer: a mortality study of compensated men with silicosis in New South Wales, Australia. Occup Med (Lond). 2004;54:387-394.
- 3. Pelucchi C, Pira E, Piolatto G, et al. Occupational silica exposure and lung cancer risk: a review of epidemiological studies 1996-2005. Ann Oncol. 2006;17:1039-1050.
- 4. Stacey P. Measurements of silica in air: reliability at new and proposed occupational exposure limits. J Occup Environ Hyg. 2007;4:D1-D4.
- 5. Flynn MR, Susi P. Engineering controls for selected silica and dust exposures in the construction industry-a review. Appl Occup Environ Hyg. 2003;18:268-277.
- 6. Verma DK, Purdham JT, Roels HA. Translating evidence about occupational conditions into strategies for prevention. Occup Environ Med. 2002;59:205-213; quiz 214.
- 7. Steenland K. One agent, many diseases: exposure-response data and comparative risks of different outcomes following silica exposure. Am J Ind Med. 2005;48:16-23.
- 8. McDonald JC, McDonald AD, Hughes JM, et al. Mortality from lung and kidney disease in a cohort of North American industrial sand workers: an update. Ann Occup Hyg. 2005;49:367-373.
- 9. Mulloy KB. Silica exposure and systemic vasculitis. Environ Health Perspect. 2003;111:1933-1938.
- 10. Steenland K, Sanderson W, Calvert GM. Kidney disease and arthritis in a cohort study of workers exposed to silica. Epidemiology. 2001;12:405-412.
- 11. Parks CG, Conrad K, Cooper GS. Occupational exposure to crystalline silica and autoimmune disease. Environ Health Perspect. 1999;(107 suppl 5):793-802.
- 12. Oliver JE, Silman AJ. Risk factors for the development of rheumatoid arthritis. Scand J Rheumatol. 2006;35:169-174.
- 13. Caplan A. Certain unusual radiological appearances in the chest of coal-miners suffering from rheumatoid arthritis. Thorax. 1953;8:29-37.
- 14. Miall WE. Rheumatoid arthritis in males; an epidemiological study of a Welsh mining community. Ann Rheum Dis. 1955;14:150-158.
- 15. Sluis-Cremer GK, Hessel PA, Hnizdo E, Churchill AR. Relationship between silicosis and rheumatoid arthritis. Thorax. 1986;41:596-601.
- 16. Schroeder W, Franklin EC, McEwen C. Rheumatoid factors in patients with silicosis with round nodular fibrosis of the lung in the absence of rheumatoid arthritis with a note on the failure to induce such factors in animals. Arthritis Rheum. 1962;5:10-18.
- 17. Jones RN, Turner-Warwick M, Ziskind M, Weill H. High prevalence of antinuclear antibodies in sandblasters’ silicosis. Am Rev Respir Dis. 1976;113:393-395.
- 18. Turner S, Cherry N. Rheumatoid arthritis in workers exposed to silica in the pottery industry. Occup Environ Med. 2000;57:443-447.
- 19. Calvert GM, Rice FL, Boiano JM, et al. Occupational silica exposure and risk of various diseases: an analysis using death certificates from 27 states of the United States. Occup Environ Med. 2003;60:122-129.
- 20. Stolt P, Kuällberg H, Lundberg I, et al. Silica exposure is associated with increased risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis. 2005;64:582-586.
- 21. Klockars M, Koskela RS, Jaärvinen E, et al. Silica exposure and rheumatoid arthritis: a follow up study of granite workers 1940-81. Br Med J (Clin Res Ed). 1987;294:997-1000.
- 22. Karlson EW, Lee IM, Cook NR, et al. A retrospective cohort study of cigarette smoking and risk of rheumatoid arthritis in female health professionals. Arthritis Rheum. 1999;42:910-917.
- 23. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1-9.
- 24. Klareskog L, Padyukov L, Lorentzen J, Alfredsson L. Mechanisms of disease: genetic susceptibility and environmental triggers in the development of rheumatoid arthritis. Nat Clin Pract Rheumatol. 2006;2:425-433.
- 25. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
- 26. Ding M, Chen F, Shi X, et al. Diseases caused by silica: mechanisms of injury and disease development. Int Immunopharmacol. 2002;2:173-182.
- 27. Vanheé D, Gosset P, Boitelle A, et al. Cytokines and cytokine network in silicosis and coal workers’ pneumoconiosis. Eur Respir J. 1995;8:834-842.
- 28. Castranova V. Signaling pathways controlling the production of inflammatory mediators in response to crystalline silica exposure: role of reactive oxygen/nitrogen species. Free Radic Biol Med. 2004;37:916-925.
- 29. Zeidler PC, Castranova V. Role of nitric oxide in pathological responses of the lung to exposure to environmental/occupational agents. Redox Rep. 2004;9:7-18.
- 30. Vallyathan V, Shi X. The role of oxygen free radicals in occupational and environmental lung diseases. Environ Health Perspect. 1997;105(suppl 1):165-177.
- 31. Green MJ, Gough AK, Devlin J, et al. Serum MMP-3 and MMP-1 and progression of joint damage in early rheumatoid arthritis. Rheumatology (Oxford). 2003;42:83-88.
- 32. Audibert F, Chedid L. Adjuvant disease induced by mycobacteria, determinants of arthritogenicity. Agents Actions. 1976;6:75-85.
- 33. Chang YH, Pearson CM, Abe C. Adjuvant polyarthritis, IV: induction by a synthetic adjuvant: immunologic, histopathologic, and other studies. Arthritis Rheum. 1980;23:62-71.
- 34. Kohashi O, Tanaka A, Kotani S, et al. Arthritis-inducing ability of a synthetic adjuvant, N-acetylmuramyl peptides, and bacterial disaccharide peptides related to different oil vehicles and their composition. Infect Immun. 1980;29:70-75.
- 35. Kleinau S, Erlandsson H, Holmdahl R, Klareskog L. Adjuvant oils induce arthritis in the DA rat, I: characterization of the disease and evidence for an immunological involvement. J Autoimmun. 1991;4:871-880.
- 36. Holmdahl R, Lorentzen JC, Lu S, et al. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol Rev. 2001;184:184-202.
- 37. Ianaro A, Cicala C, Calignano A, et al. Antivery late antigen-1 monoclonal antibody modulates the development of secondary lesion and T-cell response in experimental arthritis. Lab Invest. 2000;80:73-80.
- 38. Jacobson PB, Morgan SJ, Wilcox DM, et al. A new spin on an old model: in vivo evaluation of disease progression by magnetic resonance imaging with respect to standard inflammatory parameters and histopathology in the adjuvant arthritic rat. Arthritis Rheum. 1999;42:2060- 2073.
- 39. Halloran MM, Szekanecz Z, Barquin N, et al. Cellular adhesion molecules in rat adjuvant arthritis. Arthritis Rheum. 1996;39:810-819.
- 40. Tak PP, Gerlag DM, Aupperle KR, et al. Inhibitor of nuclear factor kappaB kinase beta is a key regulator of synovial inflammation. Arthritis Rheum. 2001;44:1897-1907.
- 41. Kuroda Y, Akaogi J, Nacionales DC, et al. Distinctive patterns of autoimmune response induced by different types of mineral oil. Toxicol Sci. 2004;78:222-228.
- 42. Lundberg I, Alfredsson L, Plato N, et al. Occupation, occupational exposure to chemicals and rheumatological disease: a register based cohort study. Scand J Rheumatol. 1994;23:305-310.
- 43. Olsson AR, Skogh T, Wingren G. Occupational determinants for rheumatoid arthritis. Scand J Work Environ Health. 2000;26:243-249.




