Article
Chronic pain update: Addressing abuse and misuse of opioid analgesics
Opioid analgesics provide effective treatment for noncancerpain, but many physicians have concerns about adverse effects,tolerance, and addiction. Misuse of these drugs is prominentin patients with chronic pain. Recognition and early prevention ofmisuse helps physicians identify the causes and proceed with patientcare. Most persons with chronic pain have a significant medicalcomorbidity (eg, asthma) that affects treatment decisions. All patientsshould undergo an initial comprehensive evaluation. Patientsoften have a psychiatric comorbidity, such as depression or anxiety.There is no gold standard for risk assessment, but several traditionalmeasures may be used. Pain medicine practitioners increasingly areusing urine drug screens to monitor adherence to long-term opioidtherapy. Controlled substance agreements help improve patient compliance.(J Musculoskel Med. 2008;25:268-277, 302)
ABSTRACT: Opioid analgesics provide effective treatment for noncancer pain, but many physicians have concerns about adverse effects, tolerance, and addiction. Misuse of these drugs is prominent in patients with chronic pain. Recognition and early prevention of misuse helps physicians identify the causes and proceed with patient care. Most persons with chronic pain have a significant medical comorbidity (eg, asthma) that affects treatment decisions. All patients should undergo an initial comprehensive evaluation. Patients often have a psychiatric comorbidity, such as depression or anxiety. There is no gold standard for risk assessment, but several traditional measures may be used. Pain medicine practitioners increasingly are using urine drug screens to monitor adherence to long-term opioid therapy. Controlled substance agreements help improve patient compliance. ( 2008;25:268-277, 302)
Chronic pain is a costly problem that influences every aspect of a person's quality of life, interfering significantly with sleep, employment, social functioning, and activities of daily living. Patients often report depression, anxiety, irritability, sexual dysfunction, and decreased energy. Family roles are altered, and worries about financial limitations and the consequences of a restricted lifestyle abound.1-5
Epidemiological studies have independently documented that chronic pain is seen as an immense problem worldwide.6-8 Symptoms affect more than 90 million Americans-about one-third of the US population. Chronic pain accounts for 21% of emergency department visits and 25% of annual missed workdays. Including both direct and indirect costs, chronic pain is responsible for up to $100 billion in annual costs, imposing the greatest economic burden of any condition.9-12
Several studies have confirmed that opioid analgesics are useful for managing acute and cancer related pain.13 They also are considered effective treatment for persons with chronic noncancer pain and are known to have a similar safety advantage to that of long-term therapy with NSAIDs. However, many physicians are reluctant to support the use of opioids in these patients because they have concerns about adverse effects, tolerance, and addiction.
Epidemiological data from the National Comorbidity Survey of Psychiatric Disorders in the United States indicate a lifetime prevalence of 7.5% for drug dependence (illicit or prescription drugs) and 14.1% for alcohol dependence.14 About 3% of Americans 18 years or older meet the criteria for illicit drug abuse or dependence as defined by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV).15 In a sample of 363 hospitalized patients, 21.8% had a current addiction to alcohol or illicit drugs.16
Prescription opioid analgesics are said to be the most frequently abused drugs in the United States, and misuse is prominent in patients with chronic pain. In a literature review, Strain17 reported that 15% to 23% of patients with chronic pain met the criteria for a substance abuse disorder.
Recognition and early prevention of substance misuse helps physicians treating patients with chronic pain identify the causes and sources and proceed with legitimate medical practice and patient care.18 Assessment and treatment protocols are needed to determine the potential for abuse when opioids are prescribed and to manage any misuse behaviors. These protocols provide physicians with a better understanding of the patient's background and behavior and help patients who show signs of medication misuse remain compliant when taking opioids for pain.
In this article, we discuss the opioid abuse and misuse issues that often arise in the treatment of patients with chronic noncancer pain and describe assessment and treatment strategies.A case report is offered to illustrate the key points (see the Box, "Case study: At risk for medication misuse," below.
Definition of terms
Concise definitions of terms help clarify the objectives of therapy with opioid analgesics (Table 1). Addiction traditionally has incorporated both physical dependence (becoming dependent on the medication and manifesting withdrawal symptoms when it is discontinued or drastically reduced) and tolerance (the need for increasing doses over time to maintain effect). More recently, it has become clear that physical dependence and tolerance are common in the use of opioids for chronic pain and are unrelated to true addiction. In this context, addiction is a behavioral pattern of substance abuse characterized by overwhelming involvement with the use of a drug.15,19 This definition focuses on compulsive use of the drug that results in physical, psychological, and social harm to the user, who continues despite this harm.
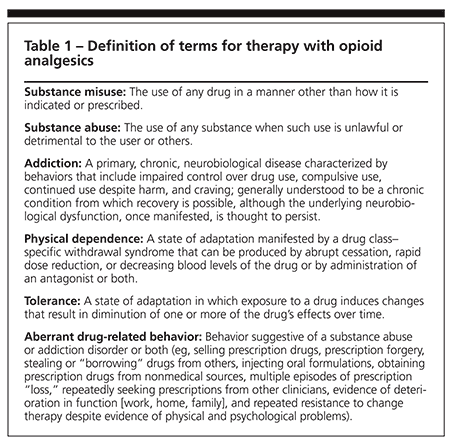
Some authors have argued that the traditional definitions are not necessarily appropriate for patients with pain who are taking opioids.20 Tolerance develops in most patients who receive long-term opioid therapy; if under-medicated, they may demonstrate drug-seeking behaviors or engage in unauthorized dosage increases. In these cases, the drug-seeking or other seemingly addictive behavior, or pseudoaddiction, disappears when an adequate analgesic dosage is reached.21
Some patients may become psychologically dependent after long-term opioid use.22,23 Others, who continue to receive extremely high doses of opioids long term, manifest impaired cognition and psychomotor performance and hyperalgesia and only show improvements in pain and neuropsychological functioning after detoxification.24 The literature points to a relationship between early misuse and addiction, which lends more support to the need for early detection and intervention.
Medical comorbidity
Most persons with chronic pain have significant medical comorbidities that affect treatment decisions. Patients often report having asthma; chronic obstructive pulmonary disease; diabetes mellitus (DM); coronary artery disease; hypertension; ulcers; kidney, bladder, and liver problems; or a history of cancer. Comorbid conditions may contribute directly to the pain complaint (eg, DM, cancer, war wounds) or may be unrelated.
Persons with chronic pain often smoke cigarettes, gain weight, and lose bone density. They may be receiving multiple medications-including blood thinners, blood pressure and heart disease medications, inhalants, and antidepressants-from multiple providers. Patients with chronic pain also are noted for allergies and reactions to some medications. They may have implanted medical devices (eg, pacemakers, rods, stimulators) or wear prostheses.
Assessing and identifying current and past medical conditions helps the treating clinician avoid complications. It also helps structure the best treatment approach.
Initial medical assessment
All patients should undergo an initial comprehensive evaluation, including a thorough medical history, review of past medical records, urine toxicology screen, and physical examination. In some cases, a psychological evaluation should be conducted, including completion of screening questionnaires. During the evaluation process, other controlled substance prescribers should be identified. Prospective patients should be made aware that providing opioid prescriptions on the first visit is unusual.
The results of the preliminary urine toxicology screen should be compared with the patient's own history of recent medication intake. A review of the patient's workup and diagnosis also should be undertaken. A diagnosis that would help explain the patient's pain should be documented. Communication should be established with the patient's other providers for appropriate coordination of management of his or her medical condition.
Psychiatric comorbidity
Patients with chronic pain often report depression, anxiety, irritability, a history of physical or sexual abuse, or a history of a mood disorder.25,26 Up to one-half of patients with chronic pain have a comorbid psychiatric condition, and 35% of patients with chronic back and neck pain have a comorbid depression or anxiety disorder. 27-29 In surveys of chronic pain clinic populations, 50% to 80% of patients had signs of psychopathology, making this the most prevalent comorbidity in these patients.30-34
In addition, patients who have chronic pain with a mood disorder are likely to have opioids prescribed for them; in one study, a 50% prevalence of mood disorder was found in these patients.35 Turk and Okifuji36 found that physicians prescribe opioids for noncancer pain more on the basis of increased affective distress and pain behavior than pain severity or objective physical pathology. Patients who have chronic pain with psychopathology also report greater pain intensity, more pain related disability, and a larger affective component to their pain than those who do not.37,38
Psychopathology also predicts a poor pain and disability outcome for patients with chronic pain, especially those with chronic low back pain.39-42 Patients who had chronic pain with anxiety and depression had a 62% worse return to work rate at 1 year than those without psychopathology.43,44 Patients who had chronic pain with low levels of psychopathology had a 40% greater reduction in pain with intravenous morphine than those in a high-psychopathology group.45 Patients with a high degree of negative affect benefited less from opioids in controlling their pain than those with a low degree.
Affective disorders often co-occur with substance use disorders, and managing a comorbid affective disorder may decrease current substance abuse behaviors or the risk of relapse.14,46-48 Some persons abuse some drugs to alleviate their psychiatric symptoms.49 This tendency to self-medicate negative affect implies that persons with a mood disorder are at increased risk for substance abuse.50 Because persons with chronic pain frequently report mood swings and prominent anxiety and depression symptoms, careful monitoring is indicated to determine who will self-medicate their mood fluctuations with medications prescribed for pain.
Substance abuse assessment
Recognizing growing support for the use of opioid analgesics in managing chronic pain, the US Department of Justice recommended efforts to improve identification of abuse and diversion of controlled substances by health care providers. 51 Physicians now are in the difficult position of trying to provide appropriate pain relief while minimizing inappropriate use of pain medications.52 Inappropriate use includes selling and diverting prescription drugs, seeking prescriptions from multiple providers, using illicit drugs, "snorting" or injecting medications, and using drugs in a manner not intended. However, there is no gold standard for assessing the risk of misuse or abuse in patients with chronic pain who are undergoing opioid treatment.
A variety of traditional assessment measures are used to identify patients with pain who are addicted, although most were developed for other purposes. The Minnesota Multiphasic Personality Inventory and related scales (eg, MacAndrew Alcoholism Scale) have been used to detect medication abuse in patients with pain, but the results have been equivocal. 53 Structured interview measures have been published for assessment of alcoholism and drug abuse based on DSM-IV criteria,54 but they have not been validated in persons with chronic pain. Other substance abuse measures include the CAGE Questionnaire, Michigan Alcoholism Screening Test, and Self-Administered Alcoholism Screening Test.55-57 Although traditional substance abuse assessment tools may be useful for persons with a severe substance abuse disorder, they are not best for persons with chronic pain because they may indicate abuse when none exists.
Other measures have been developed to screen patients with pain for addiction risk or potential. The 5-item Opioid Risk Tool, a checklist completed by the clinician, is a validated questionnaire that predicts which patients will display aberrant, drug-related behaviors.58,59 The Pain Assessment and Documentation Tool, a scale also completed by the clinician, provides a detailed means of documenting patient progress that helps record a patient's care objectively.59,60 For both tools, it helps to have some information and knowledge about the patient.

The Screener and Opioid Assessment for Patients in Pain, a 14-item self-administered screening tool developed and validated for patients with chronic pain being considered for long-term opioid therapy, is designed to predict aberrant medication-related behaviors (Table 2).61 A revised version currently is being validated.62
The Current Opioid Misuse Measure (COMM) is a 17-item questionnaire that recently was developed and validated for patients with chronic pain already receiving long-term opioid therapy. 63 Reasonably good sensitivity and specificity have established the COMM as a brief but useful self-report measure of current aberrant drug-related behavior.
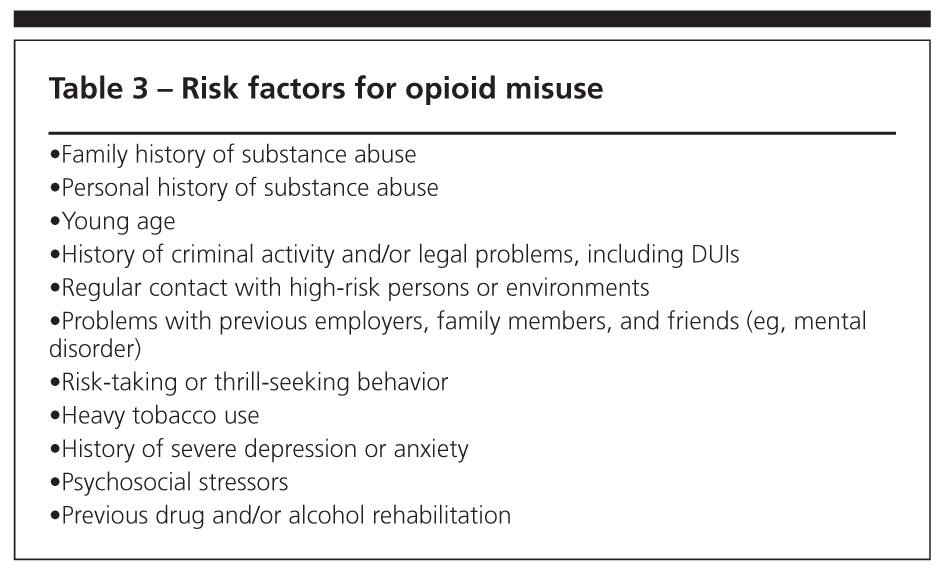
Risk factors for opioid misuse are listed inTable 3. Patients who are at lower risk for misuse include those who are older, generally compliant, and functional; show stable mood; are responsible and considerate; have a record of rarely overusing any medication; and have a likable personality. Patients who receive controlled substances should be monitored regularly to evaluate for adverse effects, continued appropriateness of therapy, and maintenance of functional gains. Repeated psychological evaluations are another aspect of appropriate follow-up care.
Urine toxicology screens
Pain medicine practitioners increasingly are using urine drug screens to monitor adherence to long-term opioid therapy. Although a urine screen by itself cannot determine the presence of addiction, a highly sensitive and specific urine screen (eg, one that uses gas chromatography/mass spectrometry) may help determine whether the patient is taking the prescribed medications, using illegal drugs, or taking non-prescribed medications. Most patients who receive long-term opioid therapy do not misuse their medication, but documenting compliance for all of these patients by obtaining a urine screen once a year is helpful.
The use of urine screens along with self-report questionnaires and behavioral observation have been valuable in determining whether a patient for whom opioids have been prescribed for pain is misusing the medication. In a study of 122 patients receiving long-term opioid therapy, urine toxicology results and aberrant drug-related behaviors identified 43% of patients as having a "problem."64 Of patients with no behavioral issues, 21% had a positive urine screen result for an illicit drug or a nonprescribed controlled medication. In another study of 226 patients with chronic pain, 46.5% of the urine toxicology screen results were abnormal.65 In yet another study, urine screen data in a retrospective study of 470 patients showed that 4 of 10 patients for whom opioids were prescribed for pain had abnormal results.66
Therefore, urine toxicology screens may be important in identifying opioid misuse. Gas chromatography/ mass spectrometry urine screens are better than immunoassays because they have a greater sensitivity to quantify illicit and prescription drugs, detect drug metabolites, and determine whether there has been any tampering with the urine sample.
Opioid therapy agreement
The use of controlled substance agreements helps improve patient compliance through education and mutual consent among patients and providers. These agreements inform patients of their responsibilities when using controlled substances and provide useful documentation for assessing compliance.
The controlled substance agreement should include patient responsibilities for self-administration of medications, medication refill intervals, pharmacy use, limiting or excluding other physicians from whom prescriptions for controlled substances may be issued, medication security, compliance with treatment plan, agreement not to use recreational drugs or prescription medications received from other persons, and consent for random drug testing (Table 4). The basic elements should include (1) taking medication as prescribed, (2) single prescription source, (3) single pharmacy, (4) no early refills, (5) no illicit substances, (6) no borrowed medication, (7) keeping scheduled appointments and actively participating in the program, and (8) random toxicology screens.

By signing an agreement, the patient acknowledges his consent to the proposed treatment plan and agreement to adhere to the specific conditions and responsibilities. Although the agreement is not seen as a legal document, failure to adhere to any of the conditions may result in the patient's discharge from treatment with opioids. It is recommended that all patients who receive long-term opioid therapy be asked to read and sign an opioid therapy agreement so that they can be informed of their responsibilities and avoid difficulties that might arise later in the therapy. Patients also may be asked to periodically complete an opioid compliance checklist to document that they have been following the opioid therapy agreement.
Universal precautions borrowed from infectious disease paradigms have been suggested as a means to identify and monitor patients who may be at risk for controlled substance abuse or diversion.67 For patients being considered for long-term opioid therapy, the following steps may be taken: (1) diagnosis with the appropriate differential; (2) psychological assessment, including risk potential for addictive disorder; (3) informed consent and treatment agreement; (4) pain and function assessment; (5) opioid therapy trial; (6) reassessment of pain, function, and behavior (eg, analgesia, activities of daily living, adverse events); (7) periodic urine screens; (8) review of diagnosis and comorbidities; and (9) detailed documentation.
Patients of primary care physicians who present with significant risk factors may benefit from referral to a comprehensive pain management center for additional assessment and input. Most patients with pain who have been prescribed a stable dose of opioids-and who in the opinion of the pain management specialists have been compliant with their opioids-can be referred back to their primary care physician for management of their pain once stability is achieved. If needed, periodic consultation and reevaluation with the pain specialists would be available.
Legal issues
Although incidents of physicians facing discipline from a medical board for overprescribing opioids for pain are rare, knowing local, state, and federal regulations and using sound clinical judgment are important. Careful monitoring of all patients for whom opioids are prescribed for pain and keeping meticulous records are crucial. Prescribing clinicians need to familiarize themselves with issues related to specifics on pain treatment and the law. For more on this topic, visit www.painandthelaw.org.
Keep detailed records of each patient visit, procedure, phone call, e-mail, and discussion. At each appointment, be sure to evaluate the pain, its location, and its intensity as well as the patient's current medication list. It is the provider's responsibility to constantly reevaluate, record, and speak with the patient about the treatment plan.
Assessment of the ongoing benefits of opioids with documented pain ratings, level of function, mood, and side effects is essential. Make sure that random regular urine screens are performed and that any abnormalities are documented and addressed immediately and appropriately. The manner in which abnormalities are addressed should be universal from patient to patient. Stay sensitive to issues of overprescribing or underprescribing opioids for pain. Using the lowest effective starting dose and systematically finding an effective drug or dose level is accepted practice. Cases have been made against physicians who allowed patients to experience pain unnecessarily.
Conclusions
Careful monitoring is indicated for all persons for whom opioids are prescribed for pain. Those with signs of high risk for misuse should be encouraged to comply with a treatment protocol that includes an opioid agreement, regular urine toxicology screens, compliance checklists, pill counts and, if indicated, motivational counseling. Future availability of opioids with low abuse potential may decrease the risk of iatrogenic addiction. However, careful attention to screening and documentation of treatment outcomes will be required.
Case study: At risk for medication misuse
Mr Smith is a 35-year-old man who injured his back when he fell from a scaffold at a construction site. MRI results showed signs of a herniated disk at L4-5. Lumbar fusion with implantation of hardware was performed, but the surgery was unsuccessful.
For the past 1½ years, Mr Smith has reported constant back pain, been out of work, and received workers' compensation. Trials of physical therapy, lumbar epidural corticosteroid injections, and nonopioid medications were unsuccessful. Eventually, long- and short-acting opioid analgesics were prescribed.
Since his injury, Mr Smith has gained weight and reported poor sleep and problems with memory and concentration. He is severely depressed, stating that he wonders whether life is worth living if he has to keep dealing with this pain. He smokes a pack of cigarettes a day, has a history of alcohol dependence, and admits to a history of anxiety and panic attacks.
Mr Smith was referred to a new primary care physician after the previous one announced that he was retiring and could no longer manage his care. Oxycodone and oxycodone with acetaminophen were prescribed for his pain. He was asked to sign an opioid agreement, complete the Screener and Opioid Assessment for Patients in Pain (SOAPP) instrument, and provide a urine sample for toxicology screening. His SOAPP score was 13 (a score of 8 or higher indicates risk of medication misuse), and the results of his toxicology screen showed traces of marijuana.
Mr Smith was informed that opioids would not be prescribed unless he showed consistently clean urine test results, that the SOAPP results indicated he was at risk for medication misuse, and that as a result, extremely careful monitoring was indicated. He was instructed to meet with a psychologist who had training in substance abuse and pain management. Methadone was prescribed for his pain, and he was given prescriptions for 2 weeks at a time. He was informed that he would be given urine screens regularly (once a month for 6 months) and that compliance with his opioid agreement was mandatory. He also was encouraged to meet with a counselor once a week and to explore vocational rehabilitation.
Mr Smith was compliant with his medication and appointments and did not violate his opioid agreement. He received multidisciplinary treatment, which included cognitive-behavioral therapy; vocational rehabilitation; and instruction in body mechanics, pacing, problem solving, and stress management. His employer was supportive in offering retraining through his insurance; eventually, he was certified as a codes inspector. Mr Smith continued to receive a steady dose of methadone for his pain and eventually was able to return to work.
References:
References
1.
Jamison RN. Comprehensive pretreatment and outcome assessment for chronic opioid therapy for nonmalignant pain.
J Pain Symptom Manage.
1996;11:231-241.
2.
Linton S. The socioeconomic impact of chronic back pain: is anyone benefiting?
Pain.
1998;75:163-168.
3.
Ohman M, Söderberg S, Lundman B. Hovering between suffering and enduring: the meaning of living with serious chronic illness.
Qual Health Res.
2003;13:528-542.
4.
Söderberg S, Strand M, Haapala M, Lundman B. Living with a women with fibromyalgia from the perspective of the husband.
J Adv Nurs.
2003;42:143-150.
5.
Otis JD, Cardella LS, Kern RD. The influence of family and culture on pain. In: Dworkin RH, Breitbart WS, eds.
Psychosocial Aspects of Pain: A Handbook for Health Care Providers.
Seattle: IASP Press; 2004:29-45.
6.
Garofalo JP, Polatin P. Low back pain: an epidemic in industrialized countries. In: Gatchel RJ, Turk DC, eds.
Psychosocial Factors in Pain: Clinical Perspectives.
New York: Guilford Press; 1999:164-174.
7.
Fordyce WE.
Back Pain in the Workplace: Management of Disability in Nonspecific Conditions.
Seattle: IASP Press; 1995.
8.
Ehrlich GE. Back pain.
J Rheumatol.
2003; 67(suppl):26-31.
9.
Frymoyer JW, Cats-Baril WL. An overview of the incidences and costs of low back pain.
Orthop Clin North Am.
1991;22:263-271.
10.
Maniadakis N, Gray A. The economic burden of back pain in the UK.
Pain.
2000;84:95-103.
11.
Ferrari R, Russell AS. Regional musculoskeletal conditions: neck pain.
Best Pract Res Clin Rheumatol.
2003;17:57-70.
12.
Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain conditions in the US workforce.
JAMA.
2003;290:2443-2454.
13.
American Academy of Pain Medicine, The American Pain Society, The American Society of Addiction Medicine. Definitions related to the use of opioids for the treatment of pain: a consensus document from the American Academy of Pain Medicine, the American Pain Society, and the American Society of Addiction Medicine. In: ASAM. Chevy Chase, MD; 2001.
14.
Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of
DSM-III-R
psychiatric disorders in the United States: results from the National Comorbidity Survey.
Arch Gen Psychiatry.
1994;51:8-19.
15.
American Psychiatric Association.
American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders.
4th ed. Washington, DC: American Psychiatric Association; 1994.
16.
Brown RL, Leonard T, Saunders LA, Papasouliotis O. The prevalence and detection of substance use disorders among inpatients ages 18-49: an opportunity for prevention.
Prev Med.
1998;27:101-110.
17.
Strain EC. Assessment and treatment of comorbid psychiatric disorders in opioid-dependent patients.
Clin J Pain.
2002(4 suppl):S14- S27.
18.
Gilson AM, Ryan KM, Joranson DE, Dahl JL. A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997-2002.
J Pain Symptom Manage.
2004;28:176-188.
19.
Leshner AI. What does it mean that addiction is a brain disease?
Monitor Psychol.
2001;32:19.
20.
Sees KL, Clark HW. Opioid use in the treatment of chronic pain: assessment of addiction.
J Pain Symptom Manage.
1993;8:257-264.
21.
Weissman DE, Haddox JD. Opioid pseudoaddiction: an iatrogenic syndrome.
Pain.
1989;36:363-366.
22.
McNairy SL, Maruta T, Ivnik RJ, et al. Prescription medication dependence and neuropsychologic function.
Pain.
1984;18:169-177.
23.
Darton LA, Dilts SL. Opioids. In: Frances RJ, Miller SL, eds.
Clinical Textbook of Addictive Disorders.
2nd ed. New York: Guilford Press; 1998:150-167.
24.
Savage SR. Addiction in the treatment of pain: significance, recognition, and management.
J Pain Symptom Manage.
1993;8:265-278.
25.
Andersson G. Epidemiological features of low back pain.
Lancet.
1999;354:581-585.
26.
Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review.
Arch Intern Med.
2003;163:2433-2445.
27.
Peloso PM, Bellamy N, Bensen W, et al. Double blind randomized placebo control trial of controlled release codeine in the treatment of osteoarthritis of the hip or knee.
J Rheumatol.
2000;27:764-771.
28.
Katz JN, Stucki G, Lipson SJ, et al. Predictors of surgical outcome in degenerative lumbar spinal stenosis.
Spine.
1999;24:2229-2233.
29.
Katz JN, Lipson SJ, Lew RA, et al. Lumbar laminectomy alone or with instrumented or noninstrumented arthrodesis in degenerative lumbar spinal stenosis: patient selection, costs, and surgical outcomes.
Spine.
1997;22:1123-1131.
30.
Caldwell JR, Hale ME, Boyd RE, et al. Treatment of osteoarthritis pain with controlled release oxycodone or fixed combination oxycodone plus acetaminophen added to nonsteroidal antiinflammatory drugs: a double blind, randomized, multicenter, placebo controlled trial.
J Rheumatol.
1999;26:862-869.
31.
Maier C, Hildebrandt J, Klinger R, et al. Morphine responsiveness, efficacy and tolerability in patients with chronic non-tumor associated pain-results of a double-blind placebo-controlled trial (MONTAS).
Pain.
2002;97:223-233.
32.
Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety.
Pain.
2004;112:372-380.
33.
Haythornthwaite JA, Menefee LA, Quatrano-Piacentini AL, Pappagallo M. Outcome of chronic opioid therapy for non-cancer pain.
J Pain Symptom Manage.
1998;15:185-194.
34.
Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind?
Pain.
2004;109:207-209.
35.
Arkinstall W, Sandler A, Goughnour B, et al. Efficacy of controlled-release codeine in chronic non-malignant pain: a randomized, placebocontrolled clinical trial.
Pain.
1995;62:169-178.
36.
Turk D, Okifuji A. What factors affect physicians’ decisions to prescribe opioids for chronic noncancer pain patients?
Clin J Pain.
1997;13:330-336.
37.
Moulin DE, Iezzi A, Amireh R, et al. Randomised trial of oral morphine for chronic non-cancer pain.
Lancet.
1996;347:143-147.
38.
Breckenridge J, Clark J. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain.
J Pain.
2003;4:344-350.
39.
Rivest C, Katz JN, Ferrante FM, Jamison RN. Effects of epidural steroid injection on pain due to lumbar spinal stenosis or herniated discs.
Arthritis Care Res.
1998;11:291-297.
40.
Rooks DS, Huang J, Bierbaum BE, et al. Effect of preoperative exercise on measures of functional status in men and women undergoing total hip and knee arthroplasty.
Arthritis Rheum.
2006;55:700-708.
41.
Rakvag TT, Klepstad P, Baar C, et al. The Val158Met polymorphism of the human catechol-
O
-methyltransferase (COMT) gene may influence morphine requirements in cancer pain patients.
Pain.
2005;116:73-78.
42.
Wasan AD, Kaptchuk TJ, Davar G, Jamison RN. The association between psychopathology and placebo analgesia in patients with discogenic low back pain.
Pain Med.
2006;7:217-228.
43.
Boersma K, Linton SJ. Screening to identify patients at risk: profiles of psychological risk factors for early intervention.
Clin J Pain.
2005;21:38-43.
44.
Fishbain DA. Approaches to treatment decisions for psychiatric comorbidity in the management of the chronic pain patient.
Med Clin North Am.
1999;83:737-760.
45.
Kong J, White NS, Kwong KK, et al. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity.
Hum Brain Mapp.
2006;27:715-721.
46.
Cornelius JR, Salloum IM, Ehler JG, et al. Fluoxetine in depressed alcoholics: a doubleblind, placebo-controlled trial.
Arch Gen Psychiatry.
1997;54:700-705.
47.
Sonne S, Brady KT. Substance abuse and bipolar comorbidity.
Psychiatr Clin North Am.
1999;22:609-627.
48.
Brady KT, Myrick H, Sonne S. Comorbid addiction and affective disorders. In: Gradam AW, Schultz TK, Wilford BB, eds.
Principles of Addiction Medicine.
2nd ed. Arlington, VA: American Society of Addiction Medicine; 1998:983-992.
49.
Hasin D, Liu X, Nunes E, et al. Effects of major depression on remission and relapse of substance dependence.
Arch Gen Psychiatry.
2002;59:375-380.
50.
Quello SB, Brady KT, Sonne S. Mood disorders and substance use disorder: a complex comorbidity.
NIDA Science Pract Prespect.
2005;3:13-24.
51.
US Department of Justice. Drug Enforcement Administration. Dispensing controlled substances for the treatment of pain. Federal Register Notices 2006; DEA-286P.
http://www.deadiversion.usdoj.gov/fed_regs/notices/2006/fr09062.htm
. Accessed May 7, 2008.
52.
Hampton T. Experts point to lessons learned from controversy over rofecoxib safety.
JAMA.
2005;293:413-414.
53.
Robinson RC, Gatchel RJ, Polatin P, et al. Screening for problematic prescription opioid use.
Clin J Pain.
2001;17:220-228.
54.
Helzer J. Psychiatric diagnoses and substance abuse in the general population: the ECA data.
NIDA Res Monogr.
1988;81:405-415.
55.
Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument.
Am J Psychiatry.
1974;131:1121-1123.
56.
Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653-1658.
57.
Swenson PA, Ives JE, Schenley RL. Photoprotection of
E coli B/r:
respiration, growth, macromolecular synthesis and repair of DNA.
Photochem Photobiol.
1975;21:235-241.
58.
Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool.
Pain Med.
2005;6:432-442.
59.
Webster LR, Dove B.
Avoiding Opioid Abuse While Managing Pain.
North Branch, MN: Sunrise River Press; 2007.
60.
Passik SD, Kirsh KL, Whitcomb L, et al. A new tool to assess and document pain outcomes in chronic pain patients receiving opioid therapy.
Clin Ther.
2004;26:552-561.
61.
Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain.
Pain.
2004;112:65-75.
62.
Butler SF, Fernandez K, Benoit C, et al. Validation of the Revised Screener and Opioid Assessment for Patients With Pain (SOAPP-R).
J Pain.
2008;9:360-372.
63.
Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure.
Pain.
2007;130:144-156.
64.
Fishbain DA, Cutler RB, Rosomoff HL, Rosomoff RS. Validity of selfreported drug use in chronic pain patients.
Clin J Pain.
1999;15:184-191.
65.
Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings.
Clin J Pain.
2007;23:173-179.
66.
Katz NP, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy.
Clin J Pain.
2002;18:S76-S82.
67.
Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain.
Pain Med.
2005;6:107-112.




