Article
Combination Pharmacological Management of Fibromyalgia
Fibromyalgia syndrome (FMS) is a complex disorder of chronic, widespread pain and tenderness usually accompanied by numerous other symptoms, including fatigue, cognitive dysfunction, nonrestorative sleep, depression, anxiety, and stiffness.
ABSTRACT: Fibromyalgia syndrome (FMS) is a complex disorder of chronic, widespread pain and tenderness usually accompanied by numerous other symptoms, including fatigue, cognitive dysfunction, nonrestorative sleep, depression, anxiety, and stiffness. Effective FMS treatment requires management of all associated symptoms. Multiple pharmacological agents often are used in combination, but patients with FMS tend to be sensitive to medications and often have difficulty in tolerating therapeutic "cocktails." Also, medications that lessen the severity of one FMS symptom may worsen the severity of others. Information on the use of combination drug therapy in FMS is limited. Although combination pharmacotherapy is a useful approach that can improve FMS management for many patients, such therapy must be given cautiously because it carries increased risks.
__________________________________________________________________________________
The 1990 American College of Rheumatology classification criteria defined fibromyalgia syndrome (FMS) exclusively by the presence of chronic widespread pain and tenderness.1 Because of this exclusivity, treatment typically focuses on pain management. However, effective FMS management requires an individualized treatment regimen that addresses not only pain but also all associated FMS symptoms, including fatigue, cognitive dysfunction ("fibrofog"), nonrestorative sleep, depression, anxiety, and stiffness.2
To improve outcomes, a symptom-based approach has been recommended that uses several pharmacological agents to manage various FMS symptoms.3 However, patients with FMS often have difficulty in tolerating such therapeutic "cocktails," and medications that lessen the severity of one FMS symptom may worsen the severity of others.
Information on combination drug therapy in FMS management is limited. In this article, I review the risks and benefits of combination pharmacotherapy in FMS management and offer treatment recommendations.
COMBINING FMS "ANCHOR" DRUGS
FMS does not respond to the classic analgesics, such as opioids and NSAIDs, because these agents do not address the root cause of FMS-dysfunctional CNS pain processing.4 Drugs that have demonstrated effectiveness in managing FMS pain, the so-called FMS anchor drugs, may work by increasing the activity of descending inhibitory pain pathways that use serotonin and norepinephrine (antidepressants, such as the serotonin-norepinephrine reuptake inhibitors [SNRIs] duloxetine and milnacipran; the tricyclic antidepressants [TCAs] amitriptyline and cyclobenzaprine; and the first-generation selective serotonin reuptake inhibitor [SSRI] fluoxetine). Or, they may decrease the activity of pain pathways by reducing excitatory neurotransmitter release (the anticonvulsant α2δ calcium channel antagonists pregabalin and gabapentin).
TABLE
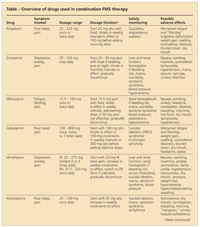
Overview of drugs used in combination FMS therapy
However, response to monotherapy usually is incomplete. There is about a 30% reduction of pain for most patients, and these modest pain improvements typically are seen with the use of high drug doses that many patients do not tolerate.
An obvious strategy for improving therapeutic effectiveness is combining anchor drugs that have different mechanisms of action (Table). In a randomized, open-label study of patients with FMS for whom pregabalin monotherapy was not successful, the addition of milnacipran, 50 mg taken twice daily, resulted in significantly more patients "much" or "very much" improved compared with those who received pregabalin maintenance therapy alone (65% vs 25%; P < .001)5; combination-treatment patients also had significantly lower pain scores. Combination therapy also improved the tolerability of adverse effects often seen with monotherapy; 13% of the combination-therapy patients experienced nausea compared with 37% for milnacipran in labeled data, and 8% of the combination-therapy group patients had dizziness compared with 38% in pregabalin registration trials.
Although combination therapy with the indicated medications is preferred, many patients cannot afford the high cost of these drugs. Because generic SNRIs and α2δ calcium channel antagonists are available, combining these agents may improve efficacy and tolerability at lower cost.
In another study, patients with neuropathy for whom gabapentin monotherapy was not successful experienced significant pain reduction and global improvement when the SNRI venlafaxine was added.6 The combination treatment was well tolerated, and patients showed reductions in many symptoms often seen in FMS, including fatigue, insomnia, and mood disturbances. However, all combination-therapy studies to date have been small, and larger studies are needed to better understand the risks and benefits.
TABLE
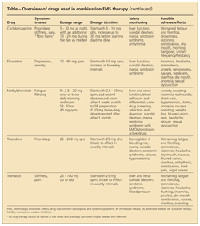
Overview of drugs used in combination FMS therapy
(continued)
Both anticonvulsants and antidepressants carry boxed warnings for suicidality. Therefore, patients who are treated with combination therapy should be monitored for suicidal thoughts, particularly those who have psychiatric disorders.
Goldenberg and associates7 found that the combination of the TCA amitriptyline, 25 mg taken at night, and the SSRI fluoxetine, 20 mg taken in the morning, works better than either drug alone for improving pain, global well-being, and sleep disturbances in patients with FMS; adverse effects were minimal. The synergy between the drugs is thought to be the result of amitriptyline increasing the norepinephrine reuptake inhibition of fluoxetine.
In another small, randomized study, the combination of fluoxetine, 20 mg/d, and cyclobenzaprine, 10 mg/d, was superior to cyclobenzaprine, 10 mg/d, alone in improving FMS pain and morning stiffness.8 Because cyclobenzaprine is a TCA similar to amitriptyline, the synergy with fluoxetine probably is the result of a mechanism similar to that of amitriptyline with fluoxetine. However, patients taking multiple serotonin-active medications are at increased risk for serotonin syndrome.9 Physicians should discuss the risks with patients and monitor them for toxicity.
ADDING MEDICATIONS TO MANAGE SPECIFIC SYMPTOMS
Although some patients have improvement of all FMS symptoms with the use of anchor combination therapy, many continue to have problematic symptoms. Drugs may be added to manage specific FMS symptoms. (Note that medication uses in this section are off-label and published data for the use of these agents in patients with FMS are limited.)
Wakefulness-promoting agents and stimulants for fatigue and fibrofog
Many patients experience fatigue that manifests physically as activity-induced exhaustion and mentally as fibrofog. Although long-term management of fatigue in patients with FMS should be based on improving sleep quality and fitness level, stimulants and wakefulness-promoting agents can improve fatigue symptoms in the short term.10
Modafinil and armodafinil are wakefulness-promoting agents indicated for daytime sleepiness associated with obstructive sleep apnea, narcolepsy, and shift work syndrome. Modafinil has shown effectiveness in improving fatigue associated with multiple disorders, including FMS,11 at doses of 50 to 400 mg given once or twice daily. Because armodafinil is the active R-enantiomer of racemic modafinil, this agent would be expected to have similar efficacy but with the added benefits of once-daily dosing, lower cost, and improved tolerability. However, the only published trial of armodafinil for FMS fatigue did not show efficacy.12
Both modafinil and armodafinil have the potential for drug interactions because they affect multiple cytochrome P-450 isoenzymes and are bound to plasma proteins. Although the adverse effects typically are milder with modafinil and armodafinil, they are similar to those seen with stimulants; patients with insomnia, cardiovascular disease, and psychiatric disorders should be monitored for symptom worsening.13
Stimulants often are used to manage FMS fatigue and fibrofog because they are inexpensive and typically there are few insurance restrictions on their use. Patients with FMS have cognitive deficits similar to those of patients with adult attention-deficit/hyperactivity disorder (ADHD),14 and adult patients who have ADHD with FMS treated with stimulants have shown improvement in multiple FMS symptoms.15
A full discussion of the risks and benefits of stimulants can be found in a recent review.10 However, a "start low and go slow" approach is recommended: a low, short-acting dose is given when the patient awakens, followed by the addition of a second dose at noon, if necessary, before a once-daily, long-acting formulation is used. For example, 5 mg of methylphenidate is taken in the morning for 1 week before adding a second dose at noon for a second week and then increasing to 10 mg twice daily for a third week before switching to a 20-mg once-daily sustained-release pill.
Because wakefulness-promoting agents and stimulants have addiction potential, their use in patients with a history of addiction is discouraged and urine drug screening is encouraged to detect diversion. When wakefulness-promoting agents and stimulants have been added to antidepressant therapy, both have been associated with serotonin syndrome and manic episodes.10
Medications for poor sleep (insomnia and nonrestorative sleep)
Disordered sleep has been implicated in the pathogenesis of FMS.16 Patients who have FMS should be evaluated for sleep disorders, and psychological and behavioral treatments should be tried before therapy with medications is considered.
Pregabalin, 25 to 225 mg taken at night, is recommended as first-line treatment for patients who have FMS with poor sleep because this agent can improve sleep and other FMS symptoms.17 Benzodiazepines are not recommended because they may have detrimental effects on sleep architecture and cognition and they have addiction potential.
Ramelteon, a melatonin receptor agonist taken as an 8-mg tablet 90 minutes before bedtime, usually is well tolerated. However, it may take 30 days to show effectiveness. Eszopiclone, a nonbenzodiazepine hypnotic taken as a 1-, 2-, or 3-mg tablet immediately before bedtime, has rapid effectiveness, but headache and an unpleasant taste can limit tolerability. Caution should be taken in combining ramelteon and eszopiclone with other sedatives, and patients should be monitored for worsening depression or suicidal ideation.
Sedating antidepressants may be beneficial for depressed patients who have FMS with insomnia. TCAs have long been used to manage nighttime pain and insomnia (5 to 30 mg of cyclobenzaprine or 10 to 50 mg of amitriptyline). Mirtazapine, 15 to 30 mg taken at night, can improve symptoms of pain, insomnia, fatigue, and depression in patients with FMS.18 Because mirtazapine somnolence is inversely proportional to dose, the dose should be increased in patients with excessive morning sedation.
Trazodone is highly sedating and best suited for patients who are refractory to other sleep medicines. In a recent open-label study of patients with FMS, trazodone monotherapy given at bedtime at an average dose of 200 mg significantly improved sleep quality along with global FMS severity and depression.19 In the same study, the subsequent addition of pregabalin to trazodone monotherapy at an average dosage of 325 mg/d led to further significant improvements in global FMS severity and depression as well as decreases in bodily pain and pain interference with activities of daily living. The adverse effects seen most frequently during the combination period, including light-headedness, dizziness, and edema, were typical of those seen with pregabalin monotherapy.
Many patients with FMS sleep well but awaken feeling unrefreshed; they require medications that improve sleep quality rather than quantity.16 Pregabalin is recommended as first-line therapy for nonrestorative sleep because it improves sleep architecture at FMS-indicated doses.17 Chlorpromazine, 100 mg taken at bedtime, has been shown to increase slow wave sleep and improve pain and mood in patients with FMS,20 but poor tolerability limits use in most patients. Quetiapine, 25 mg taken at bedtime, can improve sleep quality and other FMS symptoms,21 but tolerability is poor. Trazodone, 100 mg taken at night, improved sleep architecture in patients with somatoform pain disorder.22
Sodium oxybate, 3 mg taken at bedtime and again 4 hours later, improved sleep quality and other FMS symptoms.23 However, sodium oxybate is highly sedating and patients should have sleep testing to rule out apnea before using it. Also, because sodium oxybate has a high cost and abuse potential, it is not an option for most patients with FMS.
Managing depression and anxiety with antidepressants
One-third of patients who have FMS also have severe depression or anxiety that can hamper disease management and increase the risk of suicide.24 Therefore, management of mood disorders is vital.
Because duloxetine is FDA- approved for depression and anxiety at FMS-indicated doses, this agent is recommended as first-line therapy for patients with FMS who have mood symptoms. Venlafaxine is a generic SNRI alternative that is effective in FMS management for patients who cannot afford duloxetine.25
Milnacipran is used as an antidepressant in Europe and Japan, but monotherapy often is not sufficient to manage depression in patients with FMS and it can worsen anxiety. With the addition of a small dose of a generic SSRI to milnacipran therapy, depression and anxiety symptoms often can be managed at minimal cost with good tolerability. However, patients should be monitored for development of serotonin toxicity.9
Managing stiffness with muscle relaxants
Stiffness is problematic for most patients with FMS,1 and management is important for maintaining functionality and allowing for participation in exercise. Although daily stretching is useful for managing stiffness in all patients with FMS, muscle relaxants may be helpful adjuncts for those who have persistent stiffness.
A thorough review of the risks and benefits of muscle relaxant use is available.26 Here the focus is on the use of muscle relaxants in FMS management.
Cyclobenzaprine can improve FMS stiffness as well as pain, sleep, tenderness, and global function.27 This agent also may be helpful during FMS "flares." However, many patients cannot take cyclobenzaprine during the day because of sedation.
Amrix, an extended-release form of cyclobenzaprine, may be taken at bedtime to improve daytime tolerability. Methocarbamol and metaxalone, less sedating muscle relaxants, may be used in those patients who do not tolerate cyclobenzaprine.
Antispasmotics, such as baclofen, can reduce stiffness and manage muscle spasms, which are common in patients with FMS. Tramadol also can improve FMS stiffness.
Analgesic medications to manage FMS pain
Patients with FMS often experience pain flares that necessitate short-term analgesic therapy. Although acetaminophen is a common adjunct for FMS pain, the effective dosage usually is at the recommended maximum of 3000 mg/d and patients often are nonadherent to the necessary frequency of administration.
NSAIDs have similar analgesic effects, but efficacy in FMS management is lacking and NSAIDs are not recommended for patients with primary FMS.2 Although patients with FMS and concomitant inflammatory conditions or osteoarthritis may benefit from NSAID therapy-because such "pain generators" can worsen all FMS pain-NSAIDs combined with serotonin-active medications may increase the risk of bleeding and caution is advised in patients who are receiving antiplatelet or anticoagulant drugs or are at risk for GI bleeding.
Tramadol is the only narcotic medication recommended for managing FMS because it also has SNRI activity.2 One or 2 tramadol/acetaminophen 37.5/325-mg tablets taken 4 times daily can significantly improve pain, stiffness, and work interference in patients with FMS.28
Tapentadol, a narcotic that also has norepinephrine reuptake inhibition, currently is FDA-indicated only for relief of acute pain. Trials are needed to determine whether this agent could be beneficial in managing FMS.
Traditional narcotics should be avoided for FMS management because their effectiveness is poor and discontinuation may be difficult because of the development of rebound pain. Patients with FMS should be screened for a history of drug abuse before they receive narcotics, because abuse history is predictive of future abuse.
CONCLUSIONS
Effective FMS management requires an individualized treatment approach that addresses all problematic symptoms. Monotherapy usually is not sufficient to manage the myriad problems experienced by most patients with FMS. Although combination pharmacotherapy is a useful approach that can improve FMS management for many patients, such therapy must be given cautiously because it carries increased risks.
Problems/comments about this article? Please send feedback.
References:
References
1. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the ClassiÂfication of Fibromyalgia: report of the Multicenter CriÂteria Committee. Arthritis Rheum. 1990;33:160-172.
2. Carville SF, Arendt-Nielsen S, Bliddal H, et al; EULAR. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67:536-541.
3. Boomershine CS, Crofford LJ. A symptom-based approach to pharmacologic management of fibromyalgia. Nat Rev Rheumatol. 2009;5:191-199.
4. Russell IJ, Larson AA. Neurophysiopathogenesis of fibromyalgia syndrome: a unified hypothesis. Rheum Dis Clin North Am. 2009;35:421-435.
5. Farmer M, Dayani N, Trugman JM, et al. Efficacy of milnacipran when added to pregabalin in the management of fibromyalgia: a randomized, open-label, controlled study. Ann Rheum Dis. 2010;69(suppl 3):448.
6. Simpson DA. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. J Clin Neuromuscul Dis. 2001;3:53-62.
7. Goldenberg D, Mayskiy M, Mossey C, et al. A randomized, double-blind crossover trial of fluoxetine and amitriptyline in the treatment of fibromyalgia. Arthritis Rheum. 1996;39:1852-1859.
8. Cantini F, Bellandi F, Niccoli L, Di Munno O. FluÂoxetine combined with cyclobenzaprine in the treatment of fibromyalgia [in Italian]. Minerva Med. 1994;85:97-100.
9. Nelson LS, Erdman AR, Booze LL, et al. Selective serotonin reuptake inhibitor poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2007;45:315-332.
10. Ng B, O’Brien A. Beyond ADHD and narcolepsy: psychostimulants in general psychiatry. Adv Psychiatr Treatment. 2009;15:297-305.
11. Schwartz TL, Rayancha S, Rashid A, et al. Modafinil treatment for fatigue associated with fibromyalgia. J Clin Rheumatol. 2007;13:52.
12. Schwartz TL, Siddiqui UA, Raza S, Morell M. ArmoÂdafinil for fibromyalgia fatigue. Ann Pharmacother. 2010;44:1347-1348.
13. Kumar R. Approved and investigational uses of modafinil: an evidence-based review. Drugs. 2008;68:1803-1839.
14. Glass JM. Fibromyalgia and cognition. J Clin Psychiatry. 2008;69(suppl 2):20-24.
15. Young JL, Redmond JC. Fibromyalgia, chronic fatigue, and adult attention deficit hyperactivity disorder in the adult: a case study. Psychopharmacol Bull. 2007;40:118-126.
16. Moldofsky H. The significance, assessment, and management of nonrestorative sleep in fibromyalgia syndrome. CNS Spectr. 2008;13(3 suppl 5):22-26.
17. Hindmarch I, Dawson J, Stanley N. A double-blind study in healthy volunteers to assess the effects of sleep of pregabalin compared with alprazolam and placebo. Sleep. 2005;28:187-193.
18. Samborski W, Lezanska-Szpera M, Rybakowski JK. Open trial of mirtazapine in patients with fibromyalgia. Pharmacopsychiatry. 2004;37:168-170.
19. Calandre EP, Morillas-Arques P, Molina-Barea R, et al. Trazodone plus pregabalin combination in the treatment of fibromyalgia: a two-phase, 24-week, open-label uncontrolled study. BMC Musculoskeletal Disord. 2011;12:95. http://www.biomedcentral.com/1471-2474/12/95. Accessed August 31, 2011.
20. Moldofsky H, Lue FA. The relationship of alpha and delta EEG frequencies to pain and mood in ‘fibrositis’ patients treated with chlorpromazine and L-tryptophan. Electroencephalogr Clin Neurophysiol. 1980;50:71-80.
21. Hidalgo J, Rico-Villademoros F, Calandre EP. An open-label study of quetiapine in the treatment of fibromyalgia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:71-77.
22. Saletu B, Prause W, Anderer P, et al. Insomnia in somatoform pain disorder: sleep laboratory studies on differences to controls and acute effects of trazodone, evaluated by the Somnolyzer 24 Ã 7 and the Siesta database. Neuropsychobiology. 2005;51:148-163.
23. Moldofsky H, Inhaber NH, Guinta DR, Alvarez-Horine SB. Effects of sodium oxybate on sleep physiology and sleep/wake-related symptoms in patients with fibromyalgia syndrome: a double-blind, randomized, placebo-controlled study. J Rheumatol. 2010;37:2156-2166.
24. Ratcliffe GE, Enns MW, Belik SL, Sareen J. Chronic pain conditions and suicidal ideation and suicide attempts: an epidemiologic perspective. Clin J Pain. 2008;24:204-210.
25. Sayar K, Aksu G, Ak I, Tosun M. Venlafaxine treatment of fibromyalgia. Ann Pharmacother. 2003;37:1561-1565.
26. See S, Ginzburg R. Choosing a skeletal muscle relaxant. Am Fam Physician. 2008;78:365-370.
27. Tofferi JK, Jackson JL, O’Malley PG. Treatment of fibromyalgia with cyclobenzaprine: a meta-analysis. Arthritis Rheum. 2004;51:9-13.
28. Bennett RM, Schein J, Kosinski MR, et al. Impact of fibromyalgia pain on health-related quality of life before and after treatment with ramadol/acetaminophen. Arthritis Rheum. 2005;53:519-527.
Recommended readings
• Boomershine CS, Crofford LJ. A symptom-based approach to pharmacologic management of fibromyalgia. Nat Rev Rheumatol. 2009;5:191-199. This fibromyalgia management approach is based on the symptoms experienced by individual patients; a brief questionnaire that can be used to evaluate symptom severity is included.
• Carville SF, Arendt-Nielsen S, Bliddal H, et al; EULAR. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67:536-541. These evidence-based recommendations for fibromyalgia management include both pharmacological and nonpharmacological treatments.
• Farmer M, Dayani N, Trugman JM, et al. Efficacy of milnacipran when added to pregabalin in the management of fibromyalgia: a randomized, open-label, controlled study. Ann Rheum Dis. 2010;69(suppl 3):448. This article, the only published trial of combination therapy with indicated fibromyalgia drugs, shows that the combination of pregabalin and milnacipran improves efficacy while decreasing adverse effects.
• Nelson LS, Erdman AR, Booze LL, et al. Selective serotonin reuptake inhibitor poisoning: an evidence-based consensus guideline for out-of-hospital man-agement. Clin Toxicol (Phila). 2007;45:315-332. Evidence-based treatment guidelines for the management of serotonin syndrome in the outpatient setting are described here.
• Ng B, O’Brien A. Beyond ADHD and narcolepsy: psychostimulants in general psychiatry. Adv Psychiatr Treat. 2009;15:297-305. This reviews the stimulant therapies; readers should pay particular attention to the section on use in patients with general medical conditions.

Real-World Study Confirms Similar Efficacy of Guselkumab and IL-17i for PsA



