Article
Diabetes Cured in Lab Mice Using CRISPR Gene Editing
It's the ultimate goal for diabetes researchers: restoring the lost insulin producing function of the islet cells of the pancreas. And finally, researchers at Washington University School of Medicine in St. Louis, may be one step closer to realizing that dream.
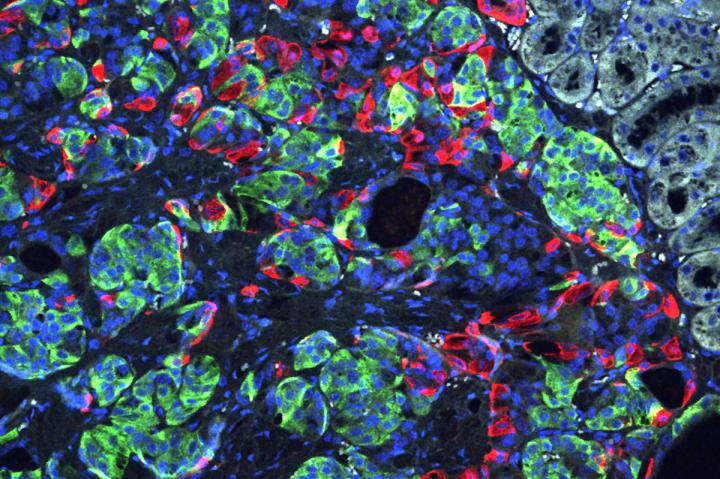
Researchers at Washington University School of Medicine in St. Louis have transformed stem cells into insulin-producing cells. They used the CRISPR gene-editing tool to correct a defect that caused a form of diabetes, and implanted the cells into mice to reverse diabetes in the animals. Shown is a microscopic image of insulin-secreting beta cells (insulin is green) that were made from stem cells produced from the skin of a patient with Wolfram syndrome. PHOTO CREDIT Millman lab Washington University
It's the ultimate goal for diabetes researchers: restoring the lost insulin producing function of the islet cells of the pancreas. And finally, researchers at Washington University School of Medicine in St. Louis, may be one step closer to realizing that dream.
Jeffrey Millman and his team induced pluripotent stem cells produced from the skin of a patient with a rare, genetic form of insulin-dependent diabetes called Wolfram syndrome. The researchers transformed the human stem cells into insulin-producing cells and used the gene-editing tool CRISPR-Cas9 to correct a genetic defect that had caused the syndrome and then implanted the cells into lab mice. The result? They cured the diabetes in those mice.
The studywas published online April 22 in the journal Science Translational Medicine.
Researchers beleive the findings suggest the CRISPR-Cas9 technique may hold promise as a treatment for diabetes, particularly the forms caused by a single gene mutation, and it also may be useful one day in some patients with the more common forms of diabetes, such as type 1 and type 2.
"This is the first time CRISPR has been used to fix a patient's diabetes-causing genetic defect and successfully reverse diabetes," said co-senior investigator Jeffrey R. Millman, PhD, an assistant professor of medicine and of biomedical engineering at Washington University. "For this study, we used cells from a patient with Wolfram syndrome because, conceptually, we knew it would be easier to correct a defect caused by a single gene. But we see this as a stepping stone toward applying gene therapy to a broader population of patients with diabetes."
In the future, using CRISPR to correct certain mutations in beta cells may help patients whose diabetes is the result of multiple genetic and environmental factors, such as type 1, caused by an autoimmune process that destroys beta cells, and type 2, which is closely linked to obesity and a systemic process called insulin resistance.
"We're excited about the fact that we were able to combine these two technologies -- growing beta cells from induced pluripotent stem cells and using CRISPR to correct genetic defects," Millman said. "In fact, we found that corrected beta cells were indistinguishable from beta cells made from the stem cells of healthy people without diabetes."
Researchers say that eventually the process of making beta cells from stem cells should get easier, and less intrusive. For example, making induced pluripotent stem cells from blood or urine. Moving forward, a patient's blood or urine sample could be used to make stem cells, and a personalized regenerative gene therapy could be customized from individualized genetic testing.
--------------
Maxwell KG, Augsornworawat P, Velazco-Cruz L, Kim MH, Asada R, Hogrebe NJ, Morikawa S, Urano F, Millman JR. Gene-edited human stem cell-derived à cells from a patient with monogenic diabetes reverse pre-existing diabetes in mice. Science Translational Medicine, published online April 22, 2020.




