Article
Equianalgesic Opioid Dosing Calculation Isn't Simple Math
Author(s):
Mary Lynn McPherson, PharmD, BCPS, CPE, FASPE, shows healthcare professionals how to apply an equianalgesic opioid dosing chart to 12 complicated patient cases requiring an opioid switch.

Though American Society of Pain Educators Board President Mary Lynn McPherson, PharmD, BCPS, CPE, FASPE, pointed out “people who do brain surgery every day look like deer in the headlights with opioid conversion … when they can ask any third grader how to do it,” her case studies featuring patients with a lack of therapeutic response and numerous adverse events and medical conditions, as well as formulations with different dose infusion rates, required skills in opioid conversion calculation above elementary school level.
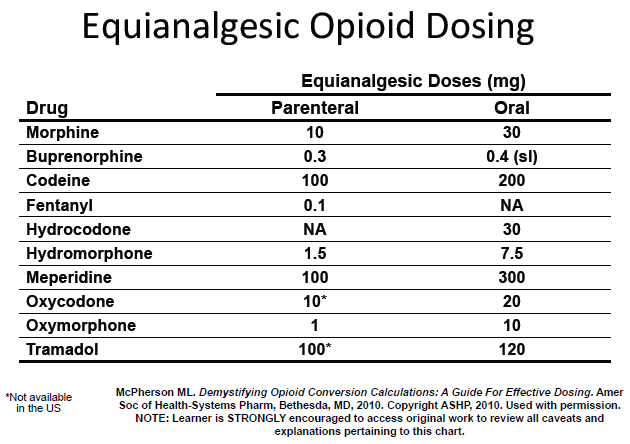
After distributing copies of an equianalgesic opioid dosing chart published in her 2008 medical book, Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing, at the start of her PAINWeek 2013 session on opioid conversions, McPherson explained that although the proportion of 30 mg of oral morphine equaling 10 mg of parenteral morphine seems straightforward, the problems with those conversion equations are that they’re accompanied by a mass of footnotes and they don’t take patient characteristics into account — which is why healthcare professionals need to complete the “five-step opioid conversion chart process” before, during, and after the switch to another analgesic.
According to McPherson, the steps of that process involve:
- Globally assessing the patient’s pain complaint.
- Determining the total daily dose of current long-acting and short-acting opioids.
- Deciding which opioid analgesic will be used as the new agent, and then referring to established conversion tables to determine the new dose.
- Individualizing the dose based on patient assessment information gathered in the first step.
- Continually reassessing the patient through seven to14 days after the initial new dose. Or, as McPherson put it, “follow your patient like it’s nobody’s business.”
Armed with the chart, the footnotes, and the steps, those in the audience were taken through a problem set of 12 case studies requiring conversions from one oral dosage formulation to another oral dosage formulation; from one route of administration to another route; from one opioid to a different opioid, regardless of opioid formulation; and to and from transdermal opioids.
Everyone arrived at the correct answer to the first case, which was that the dose of an 80-year-old man with well-controlled pain at six tablets of 5 mg oxycodone/325 mg acetaminophen per day should be converted to 5 mg oxycodone/325 mg acetaminophen per 5 ml oral solution. But by the fifth case concerning a 62-year-old woman with pancreatic cancer who was unable to swallow morphine tablets or solution, the answers started to vary.
Continuing to problem sets involving transdermal fentanyl, McPherson said it’s critical that the patient demonstrate opioid tolerance defined by the US Food and Drug Administration as 60 mg of oral morphine or equivalent dose for at least a week before the conversion, because if the patient doesn’t show that opioid tolerance and then dies from the fentanyl dose, “you can go to jail for this.”
Though McPherson referenced a conversion chart from the Butrans (buprenorphine patch) prescribing information for the final case, she noted that it’s better to avoid charts provided by pharmaceutical manufacturers because “they’re only set up for switching to their product, not for switching off of it.”
McPherson concluded her opioid conversion session by reminding healthcare providers to “be alert for clinical scenarios that may indicate opioid switching should be recommended; consider the principles of opioid responsiveness, potency, equivalence, and bioavailability; and use the five-step process.”
To learn more about using equianalgesic opioid dosing, watch this YouTube clip of McPherson:





