Article
Exercise and Physical Activity for Fibromyalgia
Multidisciplinary management of fibromyalgia syndrome, including pharmacological and nonpharmacological interventions, is optimal. Exercise may improve patients’ symptoms and quality of life, but an exercise program may be challenging because of pain, stiffness, and fatigue.
ABSTRACT: Multidisciplinary management of fibromyalgia syndrome, including pharmacological and nonpharmacological interventions, is optimal. Exercise may improve patients’ symptoms and quality of life, but an exercise program may be challenging because of pain, stiffness, and fatigue. Lifestyle physical activity involves accumulating self-selected, moderate-intensity activity over the course of the day; this may be easier to sustain over time. There is evidence for the short-term effectiveness of aerobic exercise for treatment but not for strengthening exercise. Flexibility training, yoga, and tai chi have gained increasing interest as adjunctive treatments. Patients need to develop a tolerance for the exertion associated with physical activity if it is to be effective and sustainable. (J Musculoskel Med. 2011;28:310-316)
Fibromyalgia syndrome (FMS) has no cure, and clinical management of this condition is difficult. Multidisciplinary management, including both pharmacological and nonpharmacological interventions, is optimal.1 The available FDA-approved medications for managing FMS symptoms (duloxetine, milnacipran, and pregabalin) tend to have modest and inconsistent benefits on pain, other symptoms, and quality of life.2 Nonpharmacological treatments and complementary and alternative therapies-massage therapy, psychological therapies, education, cognitive-behavioral therapy, relaxation training, and social support-may be helpful.1
Exercise also has been shown to improve patients’ symptoms and quality of life in multiple studies.3 For many patients, however, the symptom profile (eg, pain, stiffness, fatigue) makes engaging in and maintaining an exercise program challenging.
In this article, we describe the potential value of exercise and physical activity as treatment for patients with FMS. We also provide recommendations for application of the various modalities.
CHARACTERISTICS OF FMS
FMS is characterized by chronic widespread pain and multiple tender points.4 It occurs in an estimated 2% to 4% of the general US population and affects about 8 times more women than men.5 In retrospective and prospective studies, the rate of utilization of medical services was higher for patients with FMS than for other patients, patients with FMS reported more comorbid and associated conditions than other patients with rheumatologic diseases,6 and the prevalence of disability in patients with FMS was twice that of the general population.7 Preliminary diagnostic criteria now emphasize the importance of somatic symptoms associated with FMS, such as fatigue, stiffness, poor sleep, and cognitive problems.8
DEFINING EXERCISE AND PHYSICAL ACTIVITY
Physical activity is any bodily movement produced by skeletal muscles that results in energy expenditure. Exercise, a subset of physical activity, is “planned, structured, and repetitive bodily movements designed to improve or maintain physical fitness.”9 Traditionally, exercise has been categorized in 3 separate modes: aerobic exercise, strength training, and flexibility training.
A somewhat new concept, lifestyle physical activity (LPA), involves working toward accumulating at least 30 minutes of self-selected, moderate-intensity physical activity over the course of the day, 5 to 7 days per week.10 Included are physical activities performed within the household, leisure, and occupational domains (eg, doing more walking, performing more yard work, using the stairs instead of elevators, and hanging laundry).
The key element that distinguishes LPA from traditional forms of exercise is that the activities are performed in short, accumulated bouts (as little as 5 minutes each, multiple times per day) as opposed to a continuous 30- to 45-minute bout of exercise. As such, LPA is thought to be more readily integrated into the normal course of the day, making it easier to sustain over time. This may be particularly important in patients with FMS, because the fluctuating nature of their symptoms makes it more difficult to engage in, and sustain, a traditional exercise program over the long term.
FIGURE

Complementary and alternative therapies, particularly “mind-body” therapies (eg, tai chi and yoga), do not fit neatly into the conceptualizations of exercise or LPA. However, they also are being studied and applied in patients with FMS (Figure).
THE BIOLOGY OF RESPONSE TO PHYSICAL EXERTION
Understanding what appears to occur biologically when patients who have FMS engage in strenuous physical activity is important. FMS-associated pain is now widely thought to be related to dysfunctional central pain processing, which includes central sensitization and inadequate pain inhibition.11
The CNS, including the brain and the hypothalamic-pituitary-hormonal axes (HPHA), and the immune system have been implicated in pain severity in FMS. Emerging evidence suggests that there is an augmented response to normal stimuli, painful stimuli, and repetitive painful stimuli.12 In addition, patients with FMS have been found to have decreased or absent noxious inhibitory pain controls.13 Clinically, this manifests as allodynia (pain with normal stimuli) or hyperalgesia (increased pain with noxious stimuli). This, of course, has implications for the way patients with FMS respond to physical exertion.
Dysfunction of the HPHA concerning growth hormone regulation in response to exhaustive exercise correlates with increased pain scores, number of tender points, myalgic scores, and pre-exercise inflammatory cytokine levels (interleukin [IL]-1α, IL-6, and IL-8).14 The increases in cytokine levels ultimately elevate cortisol levels, possibly producing symptoms similar to those seen in “sickness behavior” (widespread pain, stiffness, fatigue, sleep disturbance, depression, and exercise intolerance).
Recent functional MRI studies showed that physical activity predicts brain responses to experimental pain.15,16 The regions of the brain involved in pain regulation were more active during physical activity; the regions associated with pain sensing were less active. Thus, it appears that pain perception is a dynamic process balanced between pain sensing and modulation that can be affected by exercise, particularly acute exercise.
Muscle along with other peripheral tissues may initiate and maintain central sensitization.11 The muscle microtrauma and repair involved in regular exercise may play a role in pain perpetuation, which may be particularly related to some metabolic findings in muscle tissue consistent with deconditioning,17 a common finding in patients who have FMS. Aerobic and strength training may improve FMS pain by normalizing some of these findings.18,19
EXERCISE AND PHYSICAL
ACTIVITY IN TREATMENT
Aerobic exercise
A 2007 Cochrane review of 34 exercise studies concluded that there was moderate-quality evidence for the short-term effectiveness of aerobic exercise for the treatment of patients with FMS (Table).20 Specifically, aerobic-only exercise training at moderate intensity levels (greater than 40% of heart rate reserve) produced positive effects on global well-being (standardized mean difference [SMD], 0.49) and physical function (SMD, 0.66). Effects on pain and tender points were variable but suggestive of benefit. The Cochrane review also concluded that evidence for the effectiveness of aerobic exercise is limited with regard to other important outcomes in FMS, such as stiffness, fatigue, and depression.20,21
TABLE
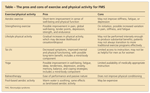
The pros and cons of exercise and physical activity for FMS
Of note, one study found that patients with lower baseline Fibromyalgia Impact Questionnaire (FIQ) scores (lower than 54/100, consistent with lower disease activity) are less likely to manifest improved functioning after an aerobic exercise intervention than those with higher baseline FIQ scores (higher than 54/100).22 In addition, patients in the aerobic exercise arm reported significantly reduced upper body pain immediately after treatment and at 3 and 9 months posttreatment compared with controls. However, there was no improvement in lower body pain.
Strengthening exercise
There is a dearth of evidence about the effectiveness of strengthening exercise for FMS. However, some small-scale studies suggested beneficial effects on pain, global well-being, tender points, and depression.20 For example, with immediate posttest evaluation of women with FMS, Kingsley and associates23 saw statistically significant improvement in upper body strength, lower body strength, endurance, subjective physical exertion, and physical function in performing everyday tasks. Still, the authors of the Cochrane review concluded that strengthening exercise cannot be recommended until the results of larger, high-quality, randomized, controlled trials have been conducted.20
Lifestyle physical activity
LPA involves accumulating 30 minutes per day of self-selected physical activities engaged in to work toward meeting the physical activity recommendations of the US Surgeon General.24 In a recent 12-week, randomized, controlled trial of 84 adults with FMS assigned to LPA or an education control, the LPA group increased their average daily steps by 54% and reduced their pain by 35% and their FIQ scores by 18%.25 However, 6- and 12-month follow-up assessments revealed that the beneficial effects of LPA on FMS symptoms were not maintained.26 Still, the patients with FMS in the LPA group did maintain a 44% increase in their average daily step count compared with baseline.
Other types of physical activity
Flexibility training, yoga, and tai chi have gained increasing interest as adjunctive treatments for patients with FMS. To date, however, few randomized trials have evaluated these exercise modalities rigorously.
Flexibility training. The effects of this modality were compared with those of strength training in a randomized trial of 68 women with FMS.27 In patients in the flexibility training arm, knee strength, shoulder strength, upper body flexibility, and symptom-related self-efficacy improved from baseline. However, the magnitude of the improvements was smaller than those obtained in the strength training arm.
Another 12-week flexibility intervention produced mild improvements in patients’ flexibility and well-being and a reduction in the number of tender points.28 However, the effects were not sustained at follow-up.
Tai chi. A recent article on the effects of tai chi-which involves components of stretching and flexibility as well as slow, controlled, rhythmic movements-strongly suggested that this form of mind-body therapy may be a viable adjunctive treatment for patients with FMS.29 In this 12-week, randomized, controlled trial, 66 patients with FMS were assigned to a tai chi group or a wellness education and stretching group (both involving two 60-minute sessions per week). After the 12 weeks, the tai chi group showed clinically and statistically significant changes on FIQ scores (18.4 units) and on the physical and mental component scores of the 36-Item Short Form Health Survey (SF-36) (changes of 7.1 and 6.1 units, respectively) compared with the controls.
On completion of the intervention, the tai chi patients were given an instructional DVD and were encouraged to continue their tai chi practice. At a follow-up assessment at 24 weeks, they had maintained their improved FIQ and SF-36 scores.
In a smaller study that focused on the stretching and flexibility components of tai chi, patients with FMS showed improvement in symptom management and quality of life after a 6-week intervention.30 In a study of men with FMS, a 4-month tai chi intervention improved lower body flexibility.31
Yoga. Like tai chi, yoga is a mind-body complementary therapy that has components of exercise; flexibility; and wellness education, particularly with regard to coping skills. In a recent 8-week pilot trial of yoga among 53 women with FMS who were randomized to a yoga program (involving gentle poses, meditation, breathing exercises, yoga-based coping instructions, and group discussions) or a weight-listed standard-care control group, the women in the yoga group showed clinically significant improvements in well-being, pain, fatigue, sleep, tenderness, depression, memory, anxiety, and balance compared with controls.32 In addition, the yoga group reported greater use of adaptive coping strategies, such as relaxation, and decreased use of maladaptive coping strategies, such as pain catastrophizing, disengagement, and acceptance.
Another study investigated more explicitly the physical components of yoga, exercise and flexibility. Improvement in well-being and pain was seen immediately after the 8-week intervention, but these benefits were not maintained at 4- to 6-week follow-up.33
Pool-based physical
activity and exercise
This appears to be a viable option for patients with FMS. The primary advantage is the often soothing effect that activities can have on achiness and tenderness, especially when they are performed in a warm-water pool. For many patients, the viscosity of the water also may reduce stiffness and muscle and joint pain.
Pool-based therapies may vary markedly in intensity, making them appropriate even for patients who have very poor tolerance for physical exertion. For example, balneotherapy (the simple immersion of the body in a mineral water bath) has been shown to decrease pain and improve function in patients with FMS.34,35
Studies have shown that pool-based exercise (walking, jumping, and some out-of-pool exercises) can produce significant improvements in patients’ physical and psychological well-being, quality of life, pain, health perceptions, balance, and capacity to climb stairs.36-38 However, lack of access to a pool, especially a warm-water pool, may be a limiting factor for many patients.
LONG-TERM ENGAGEMENT
Patients with FMS need to develop a tolerance for the exertion associated with physical activity if it is to be effective and sustainable.20 Early studies, particularly those involving aerobic exercise, often reported attrition rates in excess of 50%.3,20,21,39 Dropouts typically were attributed to the regular ebb and flow of FMS symptoms,3,20 problems in performing the exercises,40,41 and a dramatic increase in symptoms after exercise.42
The issue of attrition occurring even in well-structured, center-based clinical exercise trials speaks to the challenge of getting patients with FMS to maintain exercise regimens in their daily lives. Given the mild to moderate benefits associated with aerobic exercise and physical activity in patients who are able to adhere reasonably well,3,20 helping them develop regimens that increase the prospects of reasonable and sustainable levels of adherence seems essential.25,26 Strategies include informing patients about the risks and benefits of exercise,43 teaching them how to adjust exercises to their personal limitations,44 conveying a positive message about their physical capabilities,45 and using Web-based technologies to promote the tracking of adherence and to provide them with ongoing education and social support.26
RECOMMENDATIONS
FOR IMPLEMENTATION
Education
Because many patients prefer to use only pharmacological treatments for FMS, advising them that the medications currently available often do not effectively and completely manage the full range of symptoms is important. Clinicians should inform patients about the central nature of their allodynia and hyperalgesia and the importance of peripheral signals in their disease. With this information, patients might more readily “buy into” the potential value of a multidisciplinary treatment protocol that includes nonpharmacological therapies.
Patients also need to know that there is no “quick fix” for FMS. Clinicians should point out that partnering with a physician and other allied health professionals is essential for optimizing their functioning and quality of life.
Physical activity prescription
We often tell patients that successful FMS treatment typically includes physical activity and exercise interventions. A slow and gradual progression in frequency, duration, and intensity of physical activity may enable them to build an increasing tolerance for physical exertion that may, in turn, improve their physical function, decrease their pain and stiffness, and improve their sense of well-being.
Anticipatory education, guidance, and recommendations for “rescue” treatments are vital in helping patients cope with and manage the symptom flares that may occur as they become more physically active. We often recommend, for example, that the patient take a pain reliever before or after exercise, especially in the initial stages of physical activity, to help decrease or prevent severe postexercise pain.
As noted, patients should be advised to increase their physical activity slowly, over the course of several weeks. For example, we typically prescribe an initial accumulation of 15 minutes of LPA each day with increases of 5 minutes every 1 or 2 weeks until the patient is accumulating 30 minutes of LPA 5 to 7 days per week. We instruct patients on how to gauge whether they are performing LPA at a moderate intensity-they should be breathing heavily but should still be able to hold a conversation.
We also suggest that patients wear a pedometer to monitor their daily step counts. Self-monitoring helps many patients stay focused on the notion that they should try to maintain maximum physical activity within the context of their fluctuating symptoms. We often tell patients, “Do not overdo it when you are having a good day (a relatively symptom-free day), and do not become a couch potato when you are having a bad day (a day when your symptoms are flaring).”
As patients’ tolerance to physical exertion increases, we often suggest that they increase the variety and intensity of their physical activity to achieve greater benefit (in terms of outcomes related to FMS and general health). Indeed, for some patients, the LPA provides a gateway to the more traditional forms of exercise.
A slow, gradual introduction
of traditional exercise
It is always important to emphasize that a slow and gradual introduction of traditional exercise is prudent because it minimizes the chances of exacerbation of symptoms, which can discourage patients. Also important is conveying to patients that they are capable of becoming more physically active and that their physician will assist them in finding the most pleasant, effective, and sustainable form of physical activity or exercise that works for them.
References:
References1. Carville SF, Arendt-Nielsen S, Bliddal H, et al; EULAR. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67:536-541.
2. Gore M, Sadosky AB, Zlateva G, Clauw DJ. Clinical characteristics, pharmacotherapy and healthcare resource use among patients with fibromyalgia newly prescribed gabapentin or pregabalin. Pain Pract. 2009;9:363-374.
3. Jones KD, Liptan GL. Exercise interventions in fibromyalgia: clinical applications from the evidence. Rheum Dis Clin North Am. 2009;35:373-391.
4. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the ClassiÂfication of Fibromyalgia: report of the Multicenter CriÂteria Committee. Arthritis Rheum. 1990;33:160-172.
5. Marcus DA. Fibromyalgia: diagnosis and treatment options. Gend Med. 2009;6(suppl 2):139-151.
6. Robinson RL, Birnbaum HG, Morley MA, et al. Economic cost and epidemiological characteristics of patients with fibromyalgia claims. J Rheumatol. 2003;30:1318-1325.
7. Wolfe F, Anderson J, Harkness D, et al. A prospective, longitudinal, multicenter study of service utilization and costs in fibromyalgia. Arthritis Rheum. 1997;40:1560-1570.
8. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600-610.
9. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126-131.
10. Dunn AL, Marcus BH, Kampert JB, et al. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: a randomized trial. JAMA. 1999;281:327-334.
11. Staud R. Biology and therapy of fibromyalgia: pain in fibromyalgia syndrome. Arthritis Res Ther. 2006;8:208.
12. Staud R, Vierck CJ, Cannon RL, et al. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165-175.
13. Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189-196.
14. Ross RL, Jones KD, Bennett RM, et al. Preliminary evidence of increased pain and elevated cytokines in fibromyalgia patients with defective growth hormone response to exercise. Open Immunol J. 2010;3:9-18.
15. McLoughlin MJ, Stegner AJ, Cook DB. The relationship between physical activity and brain responses to pain in fibromyalgia. J Pain. 2011;12:640-651.
16. Cook DB, Lange G, Ciccone DS, et al. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31:364-378.
17. Elvin A, Siösteen A, Nilsson A, Kosek E. Decreased muscle blood flow in fibromyalgia patients during standardized muscle exercise: a contrast media enhanced colour Doppler study. Eur J Pain. 2006;10:137-144.
18. Deschenes MR, Kraemer WJ. Performance and physiologic adaptations to resistance training. Am J Phys Med Rehabil. 2002;81(11 suppl):S3-S16.
19. Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831-838.
20. Busch AJ, Barber KA, Overend TJ, et al. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. 2007;4:CD003786.
21. Kelley GA, Kelley KS, Hootman JM, Jones DL. Exercise and global well-being in community-dwelling adults with fibromyalgia: a systematic review with meta-analysis. BMC Public Health. 2010;10:198.
22. Da Costa D, Abrahamowicz M, Lowensteyn I, et al. A randomized clinical trial of an individualized home-based exercise programme for women with fibromyalgia. Rheumatology (Oxford). 2005;44:1422-1427.
23. Kingsley JD, Panton LB, Toole T, et al. The effects of a 12-week strength-training program on strength and functionality in women with fibromyalgia. Arch Phys Med Rehabil. 2005;86:1713-1721.
24. US Department of Health and Human Services. Physical activity and health: a report of the Surgeon General. Atlanta: US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention (CDC), National Center for Chronic Disease Prevention and Health Promotion; 1996.
25. Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity on perceived symptoms and physical function in adults with fibromyalgia: results of a randomized trial. Arthritis Res Ther. 2010;12:R55.
26. Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity in adults with fibromyalgia: results at follow-up. J Clin Rheumatol. 2011;17:64-68.
27. Jones KD, Burckhardt CS, Clark SR, et al. A randomized controlled trial of muscle strengthening versus flexibility training in fibromyalgia. J Rheumatol. 2002;29:1041-1048.
28. Valencia M, Alonso B, Alvarez MJ, et al. Effects of 2 physiotherapy programs on pain perception, muscular flexibility, and illness impact in women with fibromyalgia: a pilot study. J Manipulative Physiol Ther. 2009;32:84-92.
29. Wang C, Schmid CH, Rones R, et al. A randomized trial of tai chi for fibromyalgia. N Engl J Med. 2010;363:743-754.
30. Taggart HM, Arslanian CL, Bae S, Singh K. Effects of T’ai chi exercise on fibromyalgia symptoms and health-related quality of life. Orthop Nurs. 2003;22:353-360.
31. Carbonell-Baeza A, Romero A, Aparicio VA, et al. Preliminary findings of a 4-month tai chi intervention on tenderness, functional capacity, symptomatology, and quality of life in men with fibromyalgia. Am J Mens Health. 2011 Mar 15; [Epub ahead of print].
32. Carson JW, Carson KM, Jones KD, et al. A pilot randomized controlled trial of the Yoga of Awareness program in the management of fibromyalgia. Pain. 2010;151:530-539.
33. da Silva GD, Lorenzi-Filho G, Lage LV. Effects of yoga and the addition of Tui Na in patients with fibromyalgia. J Altern Complement Med. 2007;13:1107-1113.
34. Altan L, Bingöl U, Aykaç M, et al. Investigation of the effects of pool-based exercise on fibromyalgia syndrome. Rheumatol Int. 2004;24:272-277.
35. Evcik D, Kizilay B, Gökçen E. The effects of balneotherapy on fibromyalgia patients. Rheumatol Int. 2002;22:56-59.
36. Cedraschi C, Desmeules J, Rapiti E, et al. FibroÂmyalgia: a randomised, controlled trial of a treatment programme based on self management. Ann Rheum Dis. 2004;63:290-296.
37. Tomas-Carus P, Häkkinen A, Gusi N, et al. Aquatic training and detraining on fitness and quality of life in fibromyalgia. Med Sci Sports Exerc. 2007;39:1044-1050.
38. Martin L, Nutting A, MacIntosh BR, et al. An exercise program in the treatment of fibromyalgia. J Rheumatol. 1996;23:1050-1053.
39. Verstappen FT, van Santen-Hoeuftt HM, Bolwijn PH, et al. Effects of a group activity program for fibromyalgia patients on physical fitness and well being. J Musculoskel Pain. 1997;5:17-28.
40. van Santen M, Bolwijn P, Verstappen F, et al. A randomized clinical trial comparing fitness and biofeedback training versus basic treatment in patients with fibromyalgia. J Rheumatol. 2002;29:575-581.
41. Mannerkorpi K. Exercise in fibromyalgia. Curr Opin Rheumatol. 2005;17:190-194.
42. Mannerkorpi K, Arndorw M. Efficacy and feasibility of a combination of body awareness therapy and qigong in patients with fibromyalgia: a pilot study. J Rehabil Med. 2004;36:279-281.
43. Iversen MD, Eaton HM, Daltroy LH. How rheumatologists and patients with rheumatoid arthritis discuss exercise and the influence of discussions on exercise prescriptions. Arthritis Rheum. 2004;51:63-72.
44. Mannerkorpi K, Ahlmén M, Ekdahl C. Six- and 24-month follow up of pool exercise therapy and education for patients with fibromyalgia. Scand J Rheumatol. 2002;31:306-310.
45. Mannerkorpi K, Gard G. Physiotherapy group treatment for patients with fibromyalgia-an embodied learning process. Disabil Rehabil. 2003;25:1372-1380.

Real-World Study Confirms Similar Efficacy of Guselkumab and IL-17i for PsA



