Article
Improving Treatment Adherence in Patients With Rheumatologic Disease
Further evaluation of reasons for treatment nonadherence in patients with rheumatologic disease is key in the development of successful interventions. Patient education efforts alone are not sufficient to improve adherence; complex interventions are most effective.
In spite of advances in the understanding of treatment adherence in recent years, several issues have not been resolved. Inconsistencies in measurement of adherence among patients with rheumatologic disease often prevent comparison of study results; more accurate, validated, easy-to-use measures are needed, specifically for implementation in the clinical setting. Although these measures may need to be self-reported and therefore are subjective, additional work to establish how to inquire about medication adherence in a systematic, nonthreatening, and nonintrusive way is needed. Assessment of adherence to appointments (continuity of care) and nonmedical therapies, such as exercise, also is crucial.
Further evaluation of reasons for nonadherence in patients with rheumatologic disease is key in the development of successful interventions. What appears to be consistent across studies of chronic diseases is the notion that patient education efforts alone are not sufficient to improve adherence. Complex interventions have been most effective, but components of the interventions that will have the largest impact have yet to be identified.
Strategies for improving adherence among patients with rheumatologic disease that appear to be beneficial include the use of aids to help patients remember their regimens (eg, pill boxes, calendars) and interventions that enhance patients' self-efficacy to manage their chronic disease. Patient-centered interactions with physicians, allowing for discussion of patient preferences, also have been beneficial. In this article, we discuss various aspects of treatment adherence in patients with rheumatologic disease and strategies used to achieve it.
ADHERENCE DEFINED
Treatment adherence is defined as the extent to which patients follow recommendations and take the medications prescribed by their physicians.1-3 Ideally, patients and their physicians should agree on the recommended treatment, including duration, dosage, and frequency of medication intake over a period of time. For this to happen, patients need to be informed about their choices (ie, informed decision making). The term “medication persistence,” which has gained some favor over the past few years, refers to the maintenance of a physician's recommendations over time.4
Patients with chronic disorders are more likely to be nonadherent to treatment than those with other disease. The most frequently studied chronic disorders include HIV infection and hypertension.5
Although rheumatologic disorders have not been studied as extensively as other chronic diseases, there is evidence that nonadherence can contribute to poor long-term outcomes as well as increased utilization costs.6-9 Because most of the rheumatologic diseases require lifetime therapy, the consequences of nonadherence can be deleterious. In addition, patients with rheumatologic disease often have multiple comorbidities that require polypharmacy and a need for continuous adherence to multiple regimens. The notion that nonadherence increases with the number of pills prescribed is well recognized.10,11
MEASURING ADHERENCE
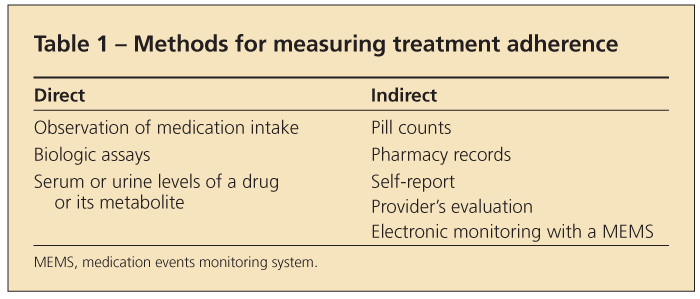
Several direct and indirect methods have been proposed to measure adherence (Table 1). Direct methods include observation of medication intake and biologic assays. Direct observation may be best suited for infusions that include a biologic agent, such as infliximab, abatacept, or rituximab, or chemotherapy, such as cyclophosphamide. Determining serum or urine levels of a drug or its metabolite also is an objective measure, but these measures are costly and subject to interval since last dose was taken and individual pharmacokinetics.12,13
A number of indirect measures of therapeutic adherence have been used in chronic diseases. They include pill counts, pharmacy records, and electronic monitoring with a medication events monitoring system (MEMS).
Pharmacy claims data have been used frequently for the study of adherence in patients with rheumatologic disease.14-19 These data can be used to measure gaps or days without medications and to estimate the medication possession ratio (the number of days for which the medication was dispensed during a specific period divided by the number of days on which the medication was expected to be taken).
Among the indirect methods, electronic monitoring provides one of the most accurate measures of adherence. However, it does not directly measure how much of the medication was ingested. Prescription drugs are dispensed in pill bottles or unit dose packages, which include a microchip that records the time and day the bottle or package was opened. Special software is used to calculate multiple adherence measures, including proportion of doses taken over a period of time, intake at correct times, and length of gaps without medication (eg, drug holidays). Although this technology often is thought of as the gold standard for promoting adherence, it is considered to be indirect because patients are not observed directly and they could open the bottle or package but not take the medication. A potential barrier to broad application of this technology is the cost.5,12,20,21
DETERMINANTS
OF ADHERENCE
Nonadherence can be intentional, as when patients purposely miss 1 or more doses of their

scheduled medications or discontinue treatment altogether, or unintentional. Patient characteristics, specifically education and socioeconomic status, have been studied in multiple chronic disease groups that were shown to be associated with nonadherence, both intentional and unintentional (Table 2).20,22
Patients' knowledge, beliefs, and attitudes can affect their intentional therapy-related behaviors, particularly those related to the potential risks and benefits of therapy. Depression, low self-efficacy, and lack of social support are other psychosocial factors that negatively influence adherence.
Adherence also is mediated through constructs of the patient-physician relationship, such as trust, provision of patient-centered care, and sensitivity to concerns and informativeness on the part of physicians.23-25 Factors associated with unintentional nonadherence include system barriers, such as drug costs, access to care, formulary coverage, and language barriers.
Forgetting to take medications at scheduled times has intentional and unintentional components-patients can engage in intentional actions to avoid missing a dose (eg, use of calendars or pill boxes). The complex interactions between patients, physicians, and the health care system generate challenges in the development and implementation of interventions to increase adherence.20
ADHERENCE IN
RHEUMATOLOGIC DISEASE
In rheumatology, therapeutic adherence has been studied most frequently in osteoporosis, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and gout. Adherence is important in these chronic conditions because they have no cure and require lifetime therapy, and a lack of adherence can have deleterious effects on patient outcomes.6-9
Osteoporosis
Overall adherence rates for patients receiving osteoporosis treatment, primarily bisphosphonates or hormonal therapy, continue to be low, even though there are multiple treatment and dosing options. Poor adherence can lead to decreased bone mineral density and an increased risk of fractures. In a meta-analysis that used claims data, more than 50% of patients were nonadherent to their bisphosphonate treatment regimen.26 Determinants of adherence in patients with osteoporosis include the dosing frequency, comorbidities, concomitant medication use, and age.26-31
Rheumatoid arthritis
In a recent systematic review of adherence in patients who had RA or SLE,22 studies were included, of which 12 assessed adherence in patients with RA.25 Most studies used indirect measures of ascertainment, often self-report. With other measures, such as pill counts, electronic monitoring, and pharmacy records, patients were considered to be adherent if they complied with the therapeutic recommendations at least 80% of the time, a cutoff that has been used widely.21,31 Overall, adherence estimates varied between 50% and 80%. Patients appeared to be more adherent with biologic therapies, particularly if given as infusions, and with methotrexate than with other, nonbiologic disease-modifying antirheumatic drugs (DMARDs).14-16
Systemic lupus
erythematosus
In reviewing adherence in patients with SLE, most studies used self-report methods to quantify it. Patients tended to be more adherent with corticosteroids than with DMARDs, particularly hydroxychloroquine.18,32
Gout
Adherence to gout medications, specifically uric acid–lowering agents (eg, allopurinol), has been measured with administrative claims data and with electronic monitoring (eg, MEMS caps). Adherence rates are reported to range from about 20% to 70%, depending on the method of adherence measurement, follow-up time, medication used, and patient characteristics.33-35 Specific characteristics of patients with gout who are more likely to be nonadherent have not been clearly identified, although some studies indicate that younger male patients who received colchicine rather than allopurinol may be the least adherent group when compared with their counterparts.33-37
INTERVENTIONS FOR
MEDICATION ADHERENCE
Multiple strategies have been used to increase medication adherence. Most have been targeted at intentional nonadherence factors, specifically patient education and medication management.
A recent Cochrane review of adherence interventions included 69 randomized controlled trials (RCTs) in which 81 interventions were assessed. Multiple medical conditions were included, not just rheumatologic disorders. Most interventions included complex methodology in which various determinants of poor adherence were targeted.
For acute conditions with short-term treatment regimens, interventions such as counseling, written instructions, and follow-up resulted in a slight increase in adherence. There was some indication of improvement in clinical outcomes, although the results were not consistent among multiple studies.
For longer-term treatments of patients with chronic diseases, results varied with respect to intervention strategies and disease-specific treatments. Overall, simplifying dosing schedules resulted in adherence improvement in most studies, although few studies assessed clinical outcomes. Simple 1-factor interventions, such as self-management techniques or the use of educational materials, had little effect on adherence or on outcomes.
Complex interventions demonstrated some improvement in both adherence and outcomes. These included combinations of educational materials; self-management plans; follow-up plans; increased interactions with a nurse, physician, or pharmacist; and the use of blister packs for medications.
Limitations in study design and confounding led to the exclusion of many RCTs from the review. A common criticism in many studies was a small sample size, which may have contributed to the lack of significant differences between intervention arms; in addition, methods of measuring adherence varied. Most studies were conducted in selected populations and controlled environments, decreasing their generalizability to community settings.38
INTERVENTIONS IN
RHEUMATOLOGIC DISEASE
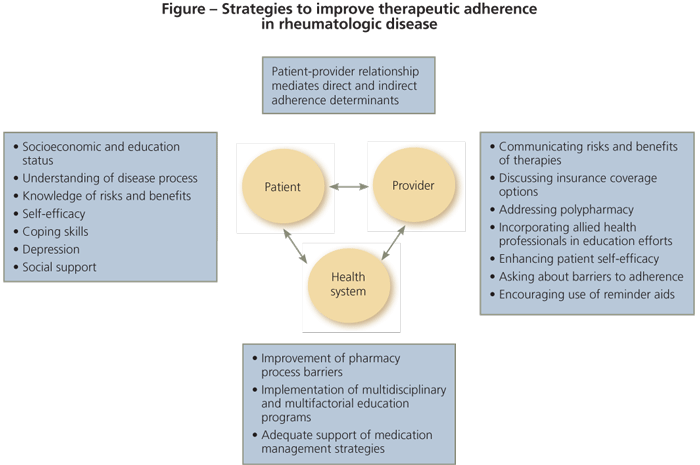
ew studies have addressed interventions for patients with rheumatologic disease specifically. Most have focused on strategies to improve intentional nonadherence. However, comparing studies is difficult because of differences in the measurement of adherence, limited data on the effects of the interventions on outcomes, limited knowledge of predictors of adherence, and varying quality of study design in complex randomized interventions. The few published studies have included patients with osteoporosis or RA. To our knowledge, no trials have targeted improving adherence in patients with SLE or gout. The Figure outlines strategies for improving adherence in rheumatologic disease.
Osteoporosis strategies
Several studies evaluated adherence strategies in osteoporosis therapy. Simple interventions, such as providing educational materials in print, did not significantly increase adherence to raloxifene therapy.39 A more complex study that involved discussions with a nurse who provided information on bone turnover markers showed improved adherence when the discussions took place compared with standard of care.40
In a similar study with a large patient population receiving personalized bone turnover marker information versus standard of care, there was an increase in treatment adherence in those who had bone turnover marker improvement (more than a 30% reduction), who then were encouraged to continue taking risedronate.41 However, overall improvement in the group receiving education alone was not demonstrated. Adherence to risedronate therapy was measured electronically with MEMS caps. Another study of an educational intervention regarding information on osteoporosis diagnosis and treatment, provided to physicians and patients, did not improve adherence significantly.42
The findings in all these studies suggest that education alone is not sufficient to improve adherence in osteoporosis and are similar to what was reported in the Cochrane review on adherence, which included various chronic diseases. As an alternative strategy, 1 study targeted patient participation in decision making about treatment by allowing patients to decide on a preferred administration mode of ibandronate therapy, oral (monthly or quarterly) or intravenous.43 Overall, adherence was higher than has been reported in other studies; it was best with less frequent dosing and intravenous administration.
Education programs for RA
Several studies have evaluated interventions to improve adherence in patients with RA. Brus and associates44 implemented a comprehensive education program with meetings that offered discussions between a trained instructor and patients about patient beliefs, problems, and solutions to treatment difficulties, and reinforcement of treatment plans. Pharmacy records and pill counts were used to assess adherence to treatment with sulfasalazine. No statistically significant difference in adherence to sulfasalazine was found between the groups who received the education intervention and those who did not.
Another study evaluated an education intervention in patients with RA that was based on self-efficacy strategies, including information about RA therapy, the disease process, and coping strategies.45 Adherence was measured with pharmacological markers, and outcomes were assessed with multiple clinical measures, including C-reactive protein level, an articular index, and morning stiffness. Patients who received the education information were significantly more adherent to their treatment regimen than their counterparts who received no additional information.
A more recent pilot study of patients with RA assessed education sessions with counselors about DMARD treatment.46 Patients were assigned to receive information individually or in groups and were given written materials about the specific drug prescribed for them along with verbal information about its risks and benefits. Adherence was measured with pill counts, self-reports, and pharmacy data. Pill counts showed that patients who received information in the group setting were more adherent to treatment.
A tailored intervention based on cognitive-behavioral therapy (CBT) incorporated multiple aspects of patient psychosocial determinants of adherence.47 Personalized individual programs targeted coping and other domains. Treatment adherence along with multiple psychosocial components increased in patients who underwent the CBT intervention compared with those who received standard care from their rheumatologist.
SUMMARY
An enabling health system coupled with multidisciplinary strategies personalized to individual patient needs can provide a positive framework for successful patient-centered interventions to improve adherence and health outcomes. Future research should evaluate the effect of these complex interventions in clinical and community settings.
References:
References
1. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44-47.
2. Harrold LR, Andrade SE. Medication adherence of patients with selected rheumatic conditions: a systematic review of the literature. Semin Arthritis Rheum. 2009;38:396-402.
3. Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826-834.
4. Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565-574; discussion 75-77.
5.Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003.
6. Bruce IN, Gladman DD, Urowitz MB. Factors associated with refractory renal disease in patients with systemic lupus erythematosus: the role of patient nonadherence. Arthritis Care Res. 2000;13:406-408.
7. Darmawan J, Rasker JJ, Nuralim H. Reduced burden of disease and improved outcome of patients with rheumatoid factor positive rheumatoid arthritis compared with dropouts: a 10 year observational study. J Rheumatol. 2003;67(suppl):S50-S53.
8. Julian LJ, Yelin E, Yazdany J, et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Rheum. 2009;61:240-246.
9. Chambers SA, Rahman A, Isenberg DA. Treatment adherence and clinical outcome in systemic lupus erythematosus. Rheumatology (Oxford). 2007;46:895-898.
10. Masood S, Jayne D, Karim Y. Beyond immunosuppression-challenges in the clinical management of lupus nephritis. Lupus. 2009;18:106-115.
11. Pons-Estel GJ, Alarcon GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39:257-268.
12. Melnikow J, Kiefe C. Patient compliance and medical research: issues in methodology. J Gen Intern Med. 1994;9:96-105.
13. Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research: a comprehensive review. J Clin Pharm Ther. 2001;26:331-342.
14. Borah BJ, Huang X, Zarotsky V, Globe D. Trends in RA patients' adherence to subcutaneous anti-TNF therapies and costs. Cur Med Res Opin. 2009;25:1365-1377.
15. Curkendall S, Patel V, Gleeson M, et al. Compliance with biologic therapies for rheumatoid arthritis: do patient out-of-pocket payments matter? Arthritis Rheum. 2008;59:1519-1526.
16. Grijalva CG, Chung CP, Arbogast PG, et al. Assessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis. Med Care. 2007;45(10 suppl 2):S66-S76.
17. Harley CR, Frytak JR, Tandon N. Treatment compliance and dosage administration among rheumatoid arthritis patients receiving infliximab, etanercept, or methotrexate. Am J Manag Care. 2003;9(6 suppl):S136-S143.
18. Koneru S, Shishov M, Ware A, et al. Effectively measuring adherence to medications for systemic lupus erythematosus in a clinical setting. Arthritis Rheum. 2007;57:1000-1006.
19. Siva C, Eisen SA, Shepherd R, et al. Leflunomide use during the first 33 months after food and drug administration approval: experience with a national cohort of 3,325 patients. Arthritis Rheum. 2003;49:745-751.
20. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
21. Hansen RA, Kim MM, Song L, et al. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43:413-422.
22. Garcia Popa-Lisseanu MG, Greisinger A, Richardson M, et al. Determinants of treatment adherence in ethnically diverse, economically disadvantaged patients with rheumatic disease. J Rheumatol. 2005;32:913-919.
23. Elliott RA. Poor adherence to medication in adults with rheumatoid arthritis: reasons and solutions. Dis Manage Health Outcomes. 2008;16:13-29.
24. Berrios-Rivera JP, Street RL Jr, Garcia Popa-Lisseanu MG, et al. Trust in physicians and elements of the medical interaction in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 2006;55:385-393.
25. de Achaval S, Suarez-Almazor ME. Treatment adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosus. Int J Clin Rheumatol. 2010;5:313-326.
26. Kothawala P, Badamgarav E, Ryu S, et al. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82:1493-1501.
27. Adachi J, Lynch N, Middelhoven H, et al. The association between compliance and persistence with bisphosphonate therapy and fracture risk: a review. BMC Musculoskelet Disord. 2007;8:97.
28. Curtis JR, Xi J, Westfall AO, et al. Improving the prediction of medication compliance: the example of bisphosphonates for osteoporosis. Med Care. 2009;47:334-341.
29. Penning-van Beest FJ, Erkens JA, Olson M, Herings RM. Determinants of non-compliance with bisphosphonates in women with postmenopausal osteoporosis. Curr Med Res Opin. 2008;24:1337-1344.
30. Warriner AH, Curtis JR. Adherence to osteoporosis treatments: room for improvement. Curr Opin Rheumatol. 2009;21:356-362.
31. Guenette L, Moisan J, Preville M, Boyer R. Measures of adherence based on self-report exhibited poor agreement with those based on pharmacy records. J Clin Epidemiol. 2005;58:924-933.
32. Koneru S, Kocharla L, Higgins GC, et al. Adherence to medications in systemic lupus erythematosus. J Clin Rheumatol. 2008;14:195-201.
33. Harrold LR, Andrade SE, Briesacher BA, et al. Adherence with urate-lowering therapies for the treatment of gout. Arthritis Res Ther. 2009;11:R46.
34. Solomon DH, Avorn J, Levin R, Brookhart MA. Uric acid lowering therapy: prescribing patterns in a large cohort of older adults. Ann Rheum Dis. 2008;67:609-613.
35. Riedel AA, Nelson M, Joseph-Ridge N, et al. Compliance with allopurinol therapy among managed care enrollees with gout: a retrospective analysis of administrative claims. J Rheumatol. 2004;31:1575-1581.
36. Halpern R, Mody RR, Fuldeore MJ, et al. Impact of noncompliance with urate-lowering drug on serum urate and gout-related healthcare costs: administrative claims analysis. Curr Med Res Opin. 2009;25:1711-1719.
37. de Klerk E, van der Heijde D, Landewe R, et al. Patient compliance in rheumatoid arthritis, polymyalgia rheumatica, and gout [published correction appears in J Rheumatol. 2003;30:423]. J Rheumatol. 2003;30:44-54.
38. Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011.
39. Guilera M, Fuentes M, Grifols M, et al; OPTIMA Study Investigators. Does an educational leaflet improve self-reported adherence to therapy in osteoporosis? The OPTIMA study. Osteoporos Int. 2006;17:664-671.
40. Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:1117-1123.
41. Delmas PD, Vrijens B, Eastell R, et al; Improving Measurements of Persistence on Actonel Treatment (IMPACT) Investigators. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis [published correction appears in J Clin Endocrinol Metab. 2007;92:2285]. J Clin Endocrinol Metab. 2007;92:1296-1304.
42. Shu AD, Stedman MR, Polinski JM, et al. Adherence to osteoporosis medications after patient and physician brief education: post hoc analysis of a randomized controlled trial. Am J Manag Care. 2009;15:417-424.
43. Lewiecki EM, Babbitt AM, Piziak VK, et al. Adherence to and gastrointestinal tolerability of monthly oral or quarterly intravenous ibandronate therapy in women with previous intolerance to oral bisphosphonates: a 12-month, open-label, prospective evaluation. Clin Ther. 2008;30:605-621.
44. Brus HL, van de Laar MA, Taal E, et al. Effects of patient education on compliance with basic treatment regimens and health in recent onset active rheumatoid arthritis. Ann Rheum Dis. 1998;57:146-151.
45. Hill J, Bird H, Johnson S. Effect of patient education on adherence to drug treatment for rheumatoid arthritis: a randomised controlled trial. Ann Rheum Dis. 2001;60:869-875.
46. Homer D, Nightingale P, Jobanputra P, et al. Providing patients with information about disease-modifying anti-rheumatic drugs: individually or in groups? A pilot randomized controlled trial comparing adherence and satisfaction. Musculoskeletal Care. 2009;7:78-92.
47. Evers AW, Kraaimaat FW, van Riel PL, de Jong AJ. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain. 2002;100:141-153.




