Publication
Article
Cardiology Review® Online
Intensification of Statin Therapy and Reduction in Atherosclerotic Inflammation
Carol L. Chen, MD
Review
HMG-CoA reductase inhibitors (statins) effectively lower serum lipid levels and decrease cardiovascular events. Antiinflammatory properties have also been attributed to the statin class due to studies evaluating their effect on circulating inflammatory markers, such as C-reactive protein (CRP). Atherosclerosis is clearly an inflammatory disorder, with progression of plaque formation involving leukocytes and macrophages. There have been many studies looking at improved outcomes in atheroma burden and clinical events beyond lipid-lowering effect with intensive statin dosing.1-3 Statins dosed intensively beyond target lipid levels to decrease CRP seem to decrease the rate of cardiac events. It is not as clear what effect statins have on the inflammatory properties of plaque.
Positron Emission Tomography-Computed Tomographic imaging (PET/CT) with 2-18F-fluoro-2-deoxy-D-glucose (FDG) has been used in both diagnosis and evaluation of treatment effect in inflammatory diseases such as Takayasu’s arteritis.4 FDG is avidly accumulated in activated macrocytes due to the high glycolytic rate. Incidental finding of increased FDG uptake on PET/CT in large vessels and corresponding to macrophage-rich carotid atheroma have been reported over the last decade.5-7 This study evaluated the feasibility of using FDG-PET/ CT to assess vascular changes in response to differing doses of atorvastatin therapy.8
Study Details
This study enrolled 83 patients across 10 US centers and 6 imaging centers. Patients were eligible for the study if they had 1 of the following:
- coronary artery disease (CAD)
- carotid disease
- cerebrovascular disease
- peripheral arterial disease, defined by abnormal ankle brachial index
- type 2 diabetes mellitus
- body mass index of 30-40 kg/m2 and waist circumference >102 cm in men and >88 cm in women
- low-density lipoprotein (LDL) cholesterol ≥60 mg/dL and triglycerides <350 mg/dL.
Patients were either on low-dose statin or were statin naïve prior to enrollment. Patients were excluded if they had 1) a significant cardiovascular event or intervention within 12 weeks of screening, 2) type 1 diabetes mellitus, 3) NYHA class III or IV congestive heart failure, 4) hepatic disease, 5) a systemic inflammatory condition, or 6) infection.
All prior statins were discontinued. Patients underwent FDGPET/ CT imaging of the carotids and ascending thoracic aorta under standard protocol, with fasting and 24-hour low-carbohydrate diet. Contrast-enhanced CT imaging was done only once either at baseline or at week 4. Forty-one patients were randomized to 80 mg of Lipitor (plus 10-mg atorvastatin matching placebo) daily and 42 patients were randomized to 10 mg Lipitor (plus 80-mg atorvastatin matching placebo) daily for 12 weeks. Twelve patients discontinued early due primarily to adverse experiences. PET/CT images were obtained again at 4 weeks and then at 12 weeks. Complete serum lipid panels and Creactive protein levels were drawn at baseline, 4, and 12 weeks.
Images were blinded and analyzed at a central core lab. Measures were taken to ensure that the same locations were measured across time. Arterial FDG uptake measured as target-to-background ratio (TBR) was evaluated in 3 locations (right and left carotid and aorta). The vessel was divided into 18 slices of approximately 3 mm. The artery with the highest FDG uptake at baseline was identified as the index vessel.
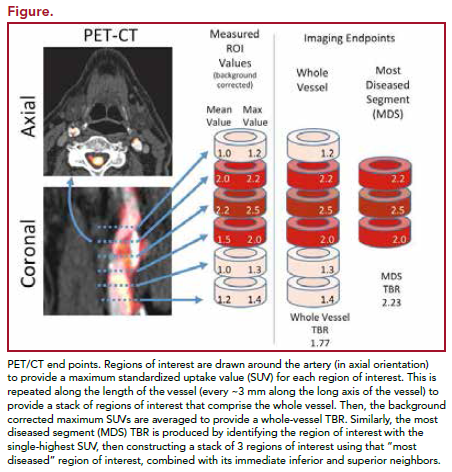
Two approaches were used to calculate index vessel FDG uptake. The first was to define the average of the maximum TBR activity within the most diseased segment (MDS) of the index vessel. The MDS was defined as the 1.5-cm arterial segment centered on the slice of artery demonstrating the highest FDG uptake at baseline, and calculated as a mean of maximum TBR values derived from 3 contiguous axial segments. The second approach was defined as the average of the maximum TBR activity for all of the axial segments that compose the index vessel (Figure). The primary objective was the change from baseline at 12 weeks in the index vessel. The secondary end point evaluated the relative change in the whole vessel TBR of the index vessel. Treatment effects in MDS and whole vessel TBR at 4 weeks, treatment effects on carotid arteries and aorta separately at 4 and 12 weeks, and relationship between lipid parameters and TBR were also studied.
After 12 weeks of treatment, there was a statistically significant reduction from baseline in the TBR within the MDS of the whole index vessel as well as the MDS in the 80-mg atorvastatin group (14.42%, P <0.001). In the group receiving 10 mg there was a small reduction in TBR MDS compared with baseline at 4 weeks, but it did not maintain statistical significance at 12 weeks (4.2%, P >0.1%). There was no significant change in TBR of whole index vessel with 10 mg atorvastatin. These results were seen as early as 4 weeks after randomization. In further analyses of carotid arteries and aorta separately, the observations remained similar to the index vessel analysis. The subset of patients who had been on low-dose statins who then received 80 mg atorvastatin still showed a significant reduction in MDS TBR at 12 weeks versus baseline.
Similar to prior statin studies, dose-dependent reductions in plasma cholesterol, LDL, and triglycerides (but not HDL) were seen. No significant correlation in individual patients was seen between changes in LDL-C or CRP and changes in TBR. This may be due to the lack of relationship of decreased inflammation to low lipid levels or because the study was not sufficiently powered to detect the relationship.
The authors concluded that their results further corroborate the significant dose-response reduction in clinical events seen in large-scale statin trials. They concluded that FDG-PET as an imaging modality of atheroma inflammation is feasible and potentially useful to assess early treatment effect in patients already taking statins.
Commentary Further Support for the Anti- Inflammatory Action of Atorvastatin
This is the first multicenter trial using FDG/PET to assess plaque activity in response to differing doses of atorvastatin over a relatively short period of time (4 to 12 weeks). The majority of previous studies of statins’ anti-inflammatory effects have concentrated on the effect on decreasing circulating inflammatory markers such as CRP. This study further supports the antiinflammatory action of atorvastatin, specifically on atheromas.
Two prior single-center FDG/PET studies demonstrated decreased FDG arterial uptake with statin treatment. Tahara et al published a single-center study randomizing patients undergoing FDG/PET for cancer screening with FDG avidity in the aorta or carotids to subsequent treatment with simvastatin or lipid-lowering diet. Simvastatin attenuated plaque inflammation significantly compared with diet alone as visualized by FDG/PET.9 A more recent single-center study using atorvastatin therapy in more modest doses (20 mg vs 5 mg) also showed reduction in arterial FDG uptake after 3 months in patients with CAD in the higher-dose group.10 This study adds to the literature by also showing a dosedependent response in the degree of inflammation as visualized by FDG/PET.
The authors acknowledge that their findings may not translate to differences in clinical events. Only large-vessel atheromas can be visualized by FDG PET/CT because myocardial uptake obscures coronary imaging. It is more reassuring that the changes were consistent in both carotids and aortas, suggesting a systemic effect. It has not yet been proved that decline in inflammatory elements of a large vessel atherosclerotic plaque would decrease acute coronary events. In the low-dose atorvastatin group there was a significant change compared with baseline at 4 weeks; however, it did not remain significantly reduced at 12 weeks. It is equally unknown whether targeting plaque inflammation over lipid-lowering goals would be beneficial.
This study adds important data to the potential uses of FDGPET/ CT in atherosclerotic inflammatory disease in that significant TBR changes were demonstrated within 4 weeks of treatment. There have been some studies suggesting that perioperative continuation of statins results in improved cardiovascular outcomes.11,12 However, the time frame in which statins should be started preoperatively for cardiovascular benefit has not been established. It would also be interesting to know how quickly the inflammatory components of plaque increase after statins are discontinued. FDGPET/ CT may add useful information in this setting as well.
The subset analysis of patients who were on low-dose statin prior to randomization to high-dose statin confirmed prior findings of incremental benefit of high-dose atorvastatin over low dose. Safety and incremental benefit of aggressive lipid lowering has been questioned.13 In PROVE-IT TIMI 2, which compared aggressive with moderate lipid lowering, all patients on strong P450 3A4 inhibitors were excluded. Despite this screening, more liver-associated side effects were seen in the high-dose atorvastatin group.2 Six patients did not complete the study because of adverse effects, but the authors do not detail whether it due to the medication or the imaging technique. It is premature to use FDG uptake to titrate statin dosing beyond current national standards since high-dose atorvastatin may cause more harm than benefit in a substantial proportion of the population. Both low and high doses of atorvastatin have been shown to reduce major cardiovascular events. The results of this study alone should not cause practitioners to increase atorvastatin doses.
This is a small cohort with short follow-up, primarily to test feasibility in a multicenter setting. It does confirm past study findings and provide more data to support use of this imaging technique in drug development and possibly efficacy if clinical events can be predicted.
References
1. Bonnet J, McPherson R, Tedgui A, et al. Comparative effects of 10-mg versus 80-mg atorvastatin on high-sensitivity C-reactive protein in patients with stable coronary artery disease: results of the CAP (Comparative Atorvastatin Pleiotropic effects) study. Clin Ther. 2008;30:2298-2313.
2. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
3. Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071-1080.
4. Zerizer I, Tan K, Khan S, et al. Role of FDG-PET and PET/CT in the diagnosis and management of vasculitis. Eur J Radiol. 2010;73:504-509.
5. Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818-1824.
6. Rudd JH, Myers KS, Bansilal S, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892-896.
7. Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708-2711.
8. Tawakol A, Fayad ZA, Mogg R, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter FDG-PET/CT feasibility study. J Am Coll Cardiol. 2013.10.1016/j .jacc.2013.04.066.
9. Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825-1831.
10. Ishii H, Nishio M, Takahashi H, et al. Comparison of atorvastatin 5 and 20 mg/d for reducing F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: a randomized, investigator-blinded, open-label, 6-month study in Japanese adults scheduled for percutaneous coronary intervention. Clin Ther. 2010;32:2337-247.
11. Raju MG, Pachika A, Punnam SR, et al. Statin therapy in the reduction of cardiovascular events in patients undergoing intermediate-risk noncardiac, nonvascular surgery [published online May 13, 2013]. Clin Cardiol. doi: 10.1002/clc.22135.
12. Poldermans D, Bax JJ, Kertai MD, et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation. 2003;107:1848-1851.
13. Davidson MH, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol. 2007;49:1753-1762.
About the Author
Carol L. Chen, MD, is Director of the Cardiac Intermediate Care Unit at Memorial Sloan-Kettering Cancer Center, New York, NY. She is board certified in cardiovascular medicine and echocardiography and specializes in cardiac disease in cancer patients. She received her MD from Weill Cornell Medical College. Dr. Chen’s residency was at NewYork-Presbyterian Hospital Center, and she was a Fellow in Cardiology at New York University Medical Center in New York, NY.
Tawakol A, Fayad ZA, Mogg R, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multi-center FDGPET/ CT feasibility study. J Am Coll Cardiol. 2013.10.1016/j.jacc.2013.04.066.