Article
Lebrikizumab Maintained Efficacy, Safety Up to a Year for Atopic Dermatitis With or Without Topical Corticosteroids
Author(s):
New late-breaking trial data from the ADhere, Adjoin, ADvocate1, and ADvocate2 trials showed solid results for lebrikizumab in treating moderate-to-severe atopic dermatitis.
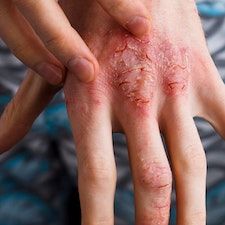
New late-breaking data presented at the 25th Annual World Congress of Dermatology (WCD) shows promising results for lebrikizumab in the maintenance treatment of moderate-to-severe atopic dermatitis (AD), with AD patients treated every 2 and 4 weeks maintaining a similar response with or without topical corticosteroids.1
The study first involved 2 trials, ADvocate1 and ADvocate2, which evaluated induction and maintenance treatment with lebrikizumab monotherapy in patients with moderate-to-severe AD.2
The third trial, ADhere, assessed induction treatment with lebrikizumab in combination with topical corticosteroids, and participants who had completed ADhere also were given the chance to roll over into ADjoin, a long-term extension study.
The objective of the ADjoin study was to assess the maintenance of both efficacy and safety in those who were able to achieve an Investigator's Global Assessment (IGA) score of 0/1 or an Eczema Area and Severity Index (EASI-75) response following 16 weeks of treatment with lebrikizumab and without rescue therapy.
The results of ADjoin indicated that the majority of patients treated with lebrikizumab every 2 weeks and every 4 weeks in ADvocate1 and ADvocate2 were shown to have maintained an IGA 0/1 response at 52 weeks, with response rates of 71.2% for the 2 week group and 76.9% for the 4 week group.
Similarly, in ADjoin, most participants given lebrikizumab plus topical corticosteroids for 2 weeks and 4 weeks were found to have maintained IGA 0/1 response at 40 weeks, with response rates of 75.4% for the 2 week group and 86.8% for the 4 week group.
Regarding the EASI-75 response, the study revealed that most patients treated with lebrikizumab Q2W and Q4W in ADvocate1 and ADvocate2 maintained their response at Week 52, with response rates of 78.4% for the 2 week group and 81.7% for 4 week group.
In ADjoin, patients treated with lebrikizumab plus TCS Q2W and Q4W maintained an EASI-75 response at Week 40, with response rates of 85.6% for Q2W and 81.2% for Q4W.
The investigators also noted that across each of the studies, the majority of lebrikizumab-treated participants also were shown to have maintained an EASI-90 response.
Additionally, safety results were found by the research team to be consistent with prior data, indicating a favorable safety profile for lebrikizumab in the maintenance treatment of the skin condition.
Lebrikizumab works as a novel, high-affinity IgG4 monoclonal antibody which inhibits IL-13 signaling to address AD.
References
- Guttman-Yassky E, Weidinger S, Simpson E, et al. Maintenance of efficacy and safety with lebrikizumab up to one year of treatment in patients with moderate-to-severe atopic dermatitis with or without topical corticosteroids. 25th Annual World Congress of Dermatology (WCD). July 2, 2023.
- Blauvelt A, Thyssen JP, Guttman-Yassky E, et al. Efficacy and safety of lebrikizumab in moderate-to-severe atopic dermatitis: 52-week results of two randomized double-blinded placebo-controlled phase III trials. Br J Dermatol. 2023;188(6):740-748. doi:10.1093/bjd/ljad022.





