Article
Managing osteoarthritis: A multidisciplinary approach
With the rising incidence of obesity and an increasing older population, the burden of osteoarthritis (OA) is expected to grow. Understanding of the disease is limited, and there are indications that many patients are receiving suboptimal care.
Osteoarthritis (OA) is the most common type of arthritis, affecting about 26.9 million persons in the United States.1 The condition is estimated to contribute $185.5 billion to annual health care expenditures in the United States,2 and with the rising incidence of obesity and an increasing older population, its burden is expected to grow.
In spite of the high prevalence of OA, understanding of the disease is limited, and currently it is an active area of research. It turns out that OA is more than just a problem of “wear and tear” of cartilage. It is a disease that involves the entire joint, which is an organ in its own right. Recently, OA has been considered as a failure of this organ, involving complex involvement of multiple structures, such as synovium, bone, nerves, and muscles. Other processes besides degeneration also are involved, including inflammation, neurological dysfunction, and mechanical dysfunction.
Not much has changed recently in the diagnosis and management of OA. Even with the treatments now available, however, there are indications that many patients are receiving suboptimal care. A study of community-dwelling elderly patients with OA noted low quality-of-care pass rates in areas such as education, exercise implementation, and medication risk counseling.3 Contributing factors might include physicians’ time limitations and tendency to gravitate toward pharmacological therapies and procedures rather than nonpharmacological therapies and education.
In this article, we provide specific diagnostic approaches and treatment guidelines for OA of the knee, hand, and hip. Our goal is to help physicians provide thorough but efficient management of OA.
DIAGNOSIS
The hallmark symptoms of OA are pain, decreased function, and limited stiffness (about 30 minutes or less of morning stiffness). Risk factors also can provide clues to the diagnosis. For example, increasing age is the strongest risk factor for practically all types of OA.1 Obesity also is a risk factor in many types of OA, especially in the weight-bearing joints. Other risk factors include trauma, surgery, excessive occupational use, and family history. In addition, inflammatory arthritides can cause joint destruction and secondary OA. Various anatomical locations have more specific guidelines for diagnosis of OA.
Knee
A diagnosis of knee OA can be made purely with clinical findings or with a combination of clinical and radiographic findings. The European League Against Rheumatism (EULAR) recommended 3 signs (crepitus, restricted range of motion, and bony enlargement) and 3 symptoms (persistent pain, limited morning stiffness, and reduced function) for making the diagnosis. The more factors present, the greater the likelihood of OA occurring. When all 6 signs and symptoms are present, the probability of radiographic knee OA is 99%.4
The American College of Rheumatology (ACR) has a similar set of purely clinical criteria that
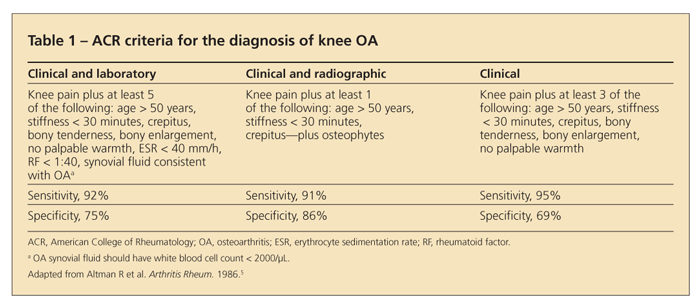
involve only the history and physical examination (Table 1).5 The ACR also provides other criteria that involve radiographs and laboratory data.
Although the history and physical examination alone often can be used to make the diagnosis, obtaining weight-bearing knee x-ray films is prudent in most cases. These films can confirm the diagnosis when there are fewer signs or symptoms; provide information on disease staging and candidacy for knee replacement; and reveal the compartments involved, which can guide the clinician in prescribing braces or insoles. Knee x-ray films also are obtained before consideration of a knee replacement, although pain and function are the primary factors that prompt the decision to undergo surgery.
Other key points. The presence of radiographic OA does not necessarily mean that the patient is symptomatic. In addition, patients with symptomatic OA may be experiencing pain resulting from other disorders. The many causes of knee pain include patellofemoral syndrome, meniscal tears, ligamentous tears, iliotibial band syndrome, and hip pain referring to the knee. The history and physical examination should be focused on ruling out these disorders.
Inflammatory signs of warmth and effusion may be present in OA, either chronic or waxing and waning. The synovial fluid white blood cell count should be less than 2000/μL. OA also is associated with gout and pseudogout, and previously damaged joints are more susceptible to septic arthritis than are normal joints. Therefore, any inflammatory flare that is out of the norm for the patient should warrant a workup for septic arthritis and crystal disease.
Hand
In many cases, a diagnosis of hand OA also can be made clinically. Symptoms and signs include hand pain with use; limited morning or inactivity stiffness; involvement of the distal interphalangeal (DIP) joints, proximal interphalangeal (PIP) joints, and base of the thumb; and the presence of Heberden and Bouchard nodes.
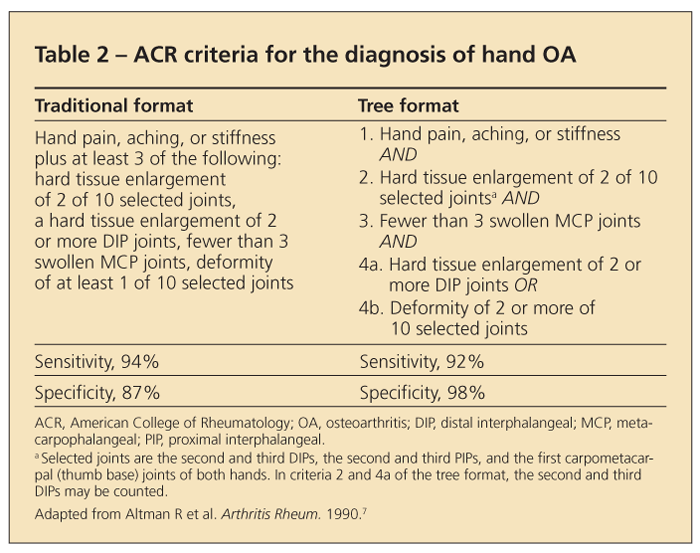
EULAR recommendations state that the probability of a patient having hand OA is 88% with the presence of the following features: patient older than 40 years, presence of Heberden nodes, family history of nodes, and joint-space narrowing in any finger joint.6 ACR guidelines have a high sensitivity and specificity (Table 2).7
Other key points. Involvement of the metacarpophalangeal (MCP) joints is uncommon in primary OA, but it can occur in the index and middle fingers. When MCP involvement is prominent, calcium pyrophosphate deposition disease, hemochromatosis, and secondary OA resulting from trauma or occupational factors should
be considered.
A less common variant of hand OA is erosive OA, in which inflammatory signs (eg, swelling, warmth, and erythema) are more prominent and the DIP and PIP joints typically are involved. Imaging findings show central erosions. Patients with erosive OA typically have greater morbidity than those with classic hand OA. Erosive OA may mimic inflammatory arthritides, such as psoriatic arthritis. When hand OA presents atypically, a rheumatology evaluation is recommended.
Hip
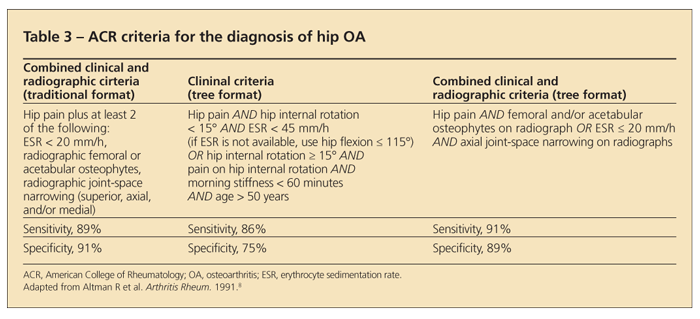
The ACR provides several criteria for the diagnosis of hip OA. All combine clinical with radiographic or laboratory features (Table 3).8
Other key points. Obtaining a specific history of hip pain from the patient is particularly important, because patients often refer to the hip as the low back or lateral trochanteric region. Lateral pain, especially with palpation, is characteristic of trochanteric bursitis. True hip pain often is felt in the groin, and it can radiate down the thigh or refer to the knee. Physical examination findings consistent with hip OA include tenderness and limited range of motion on internal rotation.
Making a diagnosis of inflammatory hip pain can be difficult. That is why laboratory and radiographic analyses are needed to help confirm the diagnosis of hip arthritis. Prominent stiffness and uniform joint-space narrowing may suggest an inflammatory arthritis, such as ankylosing spondylitis. Any acute hip pain should trigger a workup for septic arthritis, especially if it is accompanied by fever and severe tenderness on movement and weight bearing.
TREATMENT
No single treatment is considered sufficient for managing OA. The consensus is that a multifaceted approach that involves both nonpharmacological and pharmacological therapies should be used, especially for weight-bearing joints, where mechanics and lifestyle play a significant role in determining the symptoms.
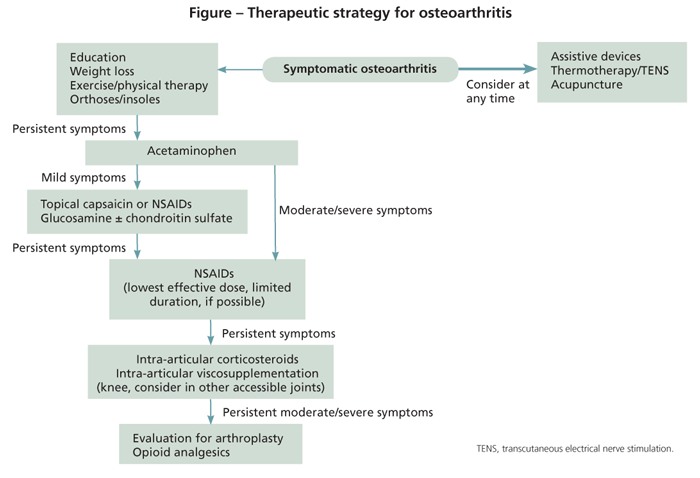
Nonpharmacological treatments should form the basis of any OA regimen, but they often are underutilized and underemphasized. The Figure shows a flowchart that summarizes the general strategy in OA management.
Nonpharmacological
Patient education. Although patient education obviously is essential, it can be especially important in OA, where much of the treatment is based on patient initiative. A thorough educational session can equip the patient with needed self-management tools.
One study showed that 80% of the cost of delivering self-care education was offset in 1 year by the decrease in the frequency and costs of primary care visits.9 Education should delineate the objectives and importance of nonpharmacological treatments and cover specific topics, such as nonpharmacological management of pain, problem solving, and principles of joint protection.10 Adjunctive methods include providing reading materials, group education, spousal education, and follow-up telephone calls.11
If time and resources are limited, high-quality education is still feasible. A physical or occupational therapist can educate the patient on nonpharmacological pain management and principles of joint protection, which may be specified in the referral request.
Orthoses for knee OA. Data on orthoses for knee OA are limited but suggest some benefit. Orthoses are recommended by major clinical guidelines on the management of knee OA.
One of the most commonly recommended types of orthoses is knee sleeves, elastic nonadhesive devices that stabilize the patella and tibiofemoral joint. A simple neoprene sleeve is easy to use and has been shown to decrease pain.12 The other common type of orthosis is unloading knee braces, custom-fitted devices that have external stems, hinges, and straps designed to decrease compressive load in the medial or lateral compartment by applying a varus or valgus force.
Studies suggest that unloading braces are more effective than knee sleeves, but treatment adherence may be an issue because unloading braces are bulkier and not as discreet as knee sleeves. Adverse effects with wear of these braces may possibly include lower extremity swelling and venous thromboembolism.13
Orthoses for hand OA. The most common reason for splinting of the hand is thumb base OA. Splints that immobilize both the wrist and the thumb base appear to be more effective than others.14 Muscle atrophy with overuse is an adverse effect. One option is to wear splints only at night; another is day use with intermittent times without splinting for strength and range of motion exercises, especially when there is occupational or activity-related discomfort. An occupational therapist should guide the fit and frequency of use of a hand splint.
Insoles and footwear for knee OA. Patients with knee OA should be evaluated for foot deformities that may affect gait and mechanical loading of the knee. The presence of foot deformities, including pes planus and hallux valgus, has been associated with increased disability in women with knee OA.15
Treatment of patients with these conditions may improve gait mechanics. In the absence of foot deformities, there may still be a benefit of insoles for knee OA.
Insoles and footwear for hip OA. Data on the effect of footwear or insoles in hip OA are limited, but experts recommend insoles and evaluation of appropriate footwear. Thus, recommendations for hip OA similar to those for knee OA would apply, including management of foot deformities and making sure patients wear functional and supportive shoes. Cushioning or neutral insoles also may be recommended, but lateral wedge insoles should be reserved for patients with medial knee OA.
Exercise for knee OA. Exercise therapy should be an integral part of OA management and has been demonstrated to improve pain and physical function. For knee OA, the effect is comparable to that reported with use of NSAIDs.16 Exercise should include both aerobic and analytic components. Aerobic exercise improves general physical performance. Analytic exercises focus on improving function of the knee with muscle strengthening, muscle and tendon lengthening, and range of motion.
Exercise for hip OA. Data on the effect of exercise in hip OA are limited, but there is consensus among major guidelines that exercise should be included in treatment. The general recommendations are similar to those for knee OA-a regimen should focus on aerobic exercise, with muscle strengthening and range of motion. The Osteoarthritis Research Society International (OARSI) states that exercising in water may be beneficial.17 A physical therapist should help create a specific regimen for each patient.
Exercise for hand OA. Exercise therapy should be used to help manage hand OA, although again data are sparse. An occupational therapist may provide guidance in creating a regimen aimed at grip strength and preserving range of motion and may recommend adaptive modalities for specific activities of daily living.
Diet/weight loss. Obesity is a risk factor for all 3 types of OA; the greatest association is with knee OA. Weight loss may improve pain and function in both knee and hip OA. A nutritional and weight loss plan should be recommended to overweight patients. No major study has examined whether weight loss improves pain in hand OA.
Other modalities. Thermotherapy may be considered for treatment of patients with knee OA, although data are limited. A Cochrane review noted that ice massage may improve range of motion and function and that cold packs may decrease swelling.18 Heat therapy is also widely used.
Other modalities that the OARSI recommends, based on reviews that show a slight but significant effect on pain, include transcutaneous electrical nerve stimulation (TENS) and acupuncture.16 Assistive walking devices, such as canes, crutches, and walkers, should be considered.
For hip OA, OARSI guidelines recommend thermotherapy and TENS, which may have a slight benefit of improving pain.17 Assistive walking devices also are recommended on the basis of expert opinion.
For hand OA, application of local heat may be used in managing pain and is recommended before exercise.14 Paraffin wax treatments often are used; paraffin wax machines may be purchased for home use at a modest cost.
PharmacologicalAcetaminophen. Recommendations for the use of pharmacological therapies are similar for all kinds of OA. Acetaminophen should be the first choice, unless there is a contraindication, because of its safety, efficacy, and tolerability. If effective, acetaminophen should be continued as the primary pharmacological treatment. Although major guidelines suggest using up to 4 g/d, the FDA recently noted concerns about the risk of liver toxicity within recommended doses.19 Educating patients about the risks of this medication is essential, and clinicians might consider lowering the maximum daily dose prescribed for patients.
NSAIDs. If acetaminophen is not sufficient, NSAIDs may be used at the lowest effective dose possible. For patients with an increased risk of GI adverse effects, a cyclo-oxygenase (COX)-2 inhibitor or nonselective NSAID should be prescribed with misoprostol or a proton pump inhibitor. Renal function should be monitored in patients taking NSAIDs for extended periods. Data about cardiovascular risk related to currently available COX-2 inhibitors are inconsistent.17
Until more definitive studies are conducted, the choice of NSAID should be made by accounting for the potential benefits and risks for each patient on the basis of his or her comorbidities. This especially applies to patients older than 70 years, in whom the risk of adverse events increases greatly. In some older patients, the judicious use of low-dose opiates may be a safer option.
Topical NSAIDs and capsaicin. These agents may be used as alternatives or adjunctive therapy to oral analgesics. They generally are safe and well-tolerated.
Intra-articular therapy. If the aforementioned therapies are not sufficiently effective or are intolerable, a trial of intra-articular therapy may be considered. The efficacy of intra-articular corticosteroids is well established; they may be used with or without signs of inflammation. They are used mostly for knee OA and to a lesser extent for hip OA, which usually requires radiological guidance. They typically are not used for hand OA, except for in the thumb base.
Intra-articular hyaluronate therapy also may be used; such therapy is characterized by a delayed onset but prolonged duration of benefit compared with intra-articular corticosteroids.17 However, controversy about its effectiveness and cost is ongoing. Hyaluronic acid derivative injections currently are FDA-approved only for use in the knee. Evidence for the benefit of hyaluronate therapy in hip OA is limited, but it can be considered.
Glucosamine and chondroitin sulfate. These agents may provide benefit in patients with knee OA. The data are heterogeneous, but because these agents have minimal adverse effects, they may be considered for patients for whom other therapies have not succeeded or for those who are intolerant to them. Of note, a recent 2-year study demonstrated no significant difference in pain reduction from glucosamine, chondroitin, or the combination, compared with placebo.20 OARSI guidelines recommend cessation after a 6-month trial proves ineffective.17
Narcotic analgesics. If the previously discussed treatments have not succeeded or are intolerable, narcotic analgesics may be used, although there have been no long-term studies on their use in OA. At this point, surgical treatment should be considered, and the use of nonpharmacological therapies should be optimized. Strong opioid analgesics should be used only in cases of extreme pain. Because many patients with OA are older, narcotics should be used with caution-their adverse effects include somnolence, constipation, and nausea.
SURGERY
If conservative treatments are not successful and if significant pain, reduced function, and decreased quality of life are present, joint replacement should be considered for patients with hip or knee OA. In patients with OA resulting from the destruction of inflammatory arthritides, function can decrease at an accelerated rate. Patients typically derive significant benefit from joint replacement.
Osteotomy may be considered for younger patients who have dysplasia or unicompartmental knee OA. The roles of joint lavage and arthroscopic debridement are controversial; a randomized controlled trial demonstrated no benefit of either procedure compared with sham surgery.21 However, there may be subsets of patients who derive benefit.
CONCLUSIONS
No therapies have been found to retard structural damage in OA, unlike for management of rheumatoid arthritis. The treatment strategies discussed here target symptoms but not the disease process itself. This is an active area of research, and as understanding of the complex pathogenic mechanisms of this disease increases, novel therapies that both improve symptoms and prevent disease progression are expected to become available.
OA is a common disease that can cause significant morbidity. Managing OA requires a multifaceted and multidisciplinary approach that combines nonpharmacological and pharmacological therapies. Physicians may have a significant effect on the quality of life of patients with OA by providing education, collaborating with other specialties, knowing the available resources, and incorporating appropriate pharmacological therapies into treatment regimens.
References:
References
1. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States, part II. Arthritis Rheum. 2008;58:26-35.
2. Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60:3546-3553.
3. Ganz DA, Chang JT, Roth CP, et al. Quality of osteoarthritis care for community-dwelling older adults. Arthritis Rheum. 2006;55:241-247.
4. Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483-489.
5. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039-1049.
6. Zhang W, Doherty M, Leeb BF, et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68:8-17.
7. Altman R, Alarcón G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33:1601-1610.
8. Altman R, Alarcón G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505-514.
9. Weinberger M, Tierney WM, Booher P, Katz BP. Can the provision of information to patients with osteoarthritis improve functional status? A randomized, controlled trial. Arthritis Rheum. 1989;32:1577-1583.
10. Mazzuca SA, Brandt KD, Katz BP, et al. Reduced utilization and cost of primary care clinic visits resulting from self-care education for patients with osteoarthritis of the knee. Arthritis Rheum. 1999;42:1267-1273.
11. Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-1155.
12. Beaudreuil J, Bendaya S, Faucher M, et al. Clinical practice guidelines for rest orthosis, knee sleeves, and unloading knee braces in knee osteoarthritis. Joint Bone Spine. 2009;76:629-636.
13. Giori NJ. Load-shifting brace treatment for osteoarthritis of the knee: a minimum 2½-year follow-up study. J Rehabil Res Dev. 2004;41:187-194.
14. Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2007;66:377-388.
15. Guler H, Karazincir S, Turhanoglu AD, et al. Effect of coexisting foot deformity on disability in women with knee osteoarthritis. J Am Podiatr Med Assoc. 2009;99:23-27.
16. Rannou F, Poiraudeau S. Non-pharmacological approaches for the treatment of osteoarthritis. Best Pract Res Clin Rheumatol. 2010;24:93-106.
17. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;
16:137-162.
18. Brosseau L, Yonge KA, Robinson V, et al. Thermotherapy for treatment of osteoarthritis. Cochrane Database Syst Rev. 2003(4):CD004522.
19. Food and Drug Administration, HHS. Organ-specific warnings; internal analgesic, antipyretic, and antirheumatic drug products for over-the-counter human use; final monograph. Final rule. Fed Regis. 2009;74:19385-19409.
20. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulfate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
21. Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis




