Article
Managing RA in 2011: An update
With the advent of expensive laboratory tests and sophisticated imaging, the diagnosis of rheumatoid arthritis (RA) remains clinical. Because early detection is important for preventing clinical and radiographic progression, revision of the diagnostic criteria was needed and has been implemented.
Rheumatoid arthritis (RA) is one of the most common immune-mediated chronic inflammatory arthritides, affecting between 0.5% and 1% of the world's population.1 In addition to causing joint-specific signs and symptoms, such as pain, stiffness, swelling, and destruction of cartilage and bone, RA is associated with many extra-articular manifestations, such as pleurisy, pericarditis, pulmonary fibrosis, nodulosis and, rarely, vasculitis and renal amyloidosis, in the later stages. The disease burden is tremendous, including substantial disability, with a high financial cost.2
Early diagnosis and aggressive treatment strategies have greatly improved outcomes for patients with RA within the past decade. In this article, we review the current approaches to diagnosis and management of this complex and challenging disease.
DIAGNOSIS
Even in an era of expensive laboratory tests and sophisticated imaging, the diagnosis of RA remains clinical. Until recently, the diagnostic criteria for RA were polyarthritis involving the small joints of the hands and feet, positive rheumatoid factor, rheumatoid nodules, and radiographic changes.3 However, the sensitivity of these features is limited in early disease, because the last 2 criteria are unlikely to be present until later in the disease course and polyarthritis also may not manifest until later. Because early detection is important for preventing clinical and radiographic progression, revision of these criteria was needed and recently implemented.
In 2010, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) copublished a new criteria set for RA. The goals included facilitating earlier diagnosis with the intent of initiation of treatment and identifying patients at high risk for progressive disease with erosive damage who may be candidates for disease-modifying therapy. The new criteria were developed on the basis of studying cohorts of diverse nationalities and backgrounds; as a result, generalizability may be enhanced.4
The new ACR/EULAR criteria also define a target population of patients whose presenting symptoms create enough suspicion to warrant further evaluation. These are patients who have definite clinical synovitis in at least 1 joint that is not explained by another disease. Also incorporated, in addition to rheumatoid factor, were anticyclic citrullinated peptide (anti-CCP) antibody, which is more specific for RA, and acute phase reactants.4
TREATMENTGeneral principles
The early paradigm for treating patients with RA in the mid 20th century was a pyramid approach that used a base of physical modalities. The next steps of the pyramid consisted of NSAIDs, gold, D-penicillamine, and an apex of azathioprine and cyclophosphamide that represented “last resort” options. Corticosteroids also were incorporated into the pyramid as adjuncts. Lack of robust evidence and fear of toxicity limited the use of available disease-modifying agents.
This pyramid now is obsolete and has been inverted. Much evidence has shown that improving outcomes requires more, rather than less, aggressive therapy early on. The change in paradigm was modeled on cancer chemotherapy, aiming for remission and frequently combining several agents to achieve it.5 However, one of the challenges in RA is a lack of universal agreement on a definition of remission, which remains an active area of discussion and research.6 Until such agreement is reached, treating patients to an objective target is preferred.
For example, intensive treatment targeting a low disease activity score (the 28-joint Disease Activity Score) was shown in a randomized controlled trial to result in significantly improved response and rate of remission.7 In addition, the intensively treated patients had improved functionality and quality of life, and the improvements came at no extra cost, without the use of tumor necrosis factor α (TNF-α) inhibitors.

Five-year follow-up data also indicated that the intensively treated patients had less radiographic progression, and clinical benefits were maintained even when patients returned to usual therapy.8Figure 1 highlights the parameters that are useful in determining therapy and tailoring it on the basis of disease activity and other prognostic factors.9
Nonpharmacological therapy
Numerous nonpharmacological therapies are available for RA. Randomized controlled data support the use of exercise and self-management programs; the evidence is more modest for joint protection, orthoses, and comprehensive care interventions and is weak for massage and electrophysical modalities.10
Pharmacological therapy
Controlling inflammation and achieving and maintaining remission in RA requires pharmacological therapy. Immunological processes in RA are known to precede clinical manifestations by many years. In the early stages of RA, the functional loss is more dependent on disease activity; in the later stages, functional loss corresponds with structural damage. Therefore, the window of opportunity to prevent functional loss may occur early in the disease course.
The available data strongly support treating patients who have RA as early as possible with disease-modifying antirheumatic drugs (DMARDs). Early treatment results in improvement of symptoms and signs and prevents radiographic progression. Comparison of the efficacy and toxicity of DMARDs and NSAIDs unequivocally favors early DMARD use.11 For this reason, NSAIDs are being used less and less, although they may help ameliorate joint pain and swelling.
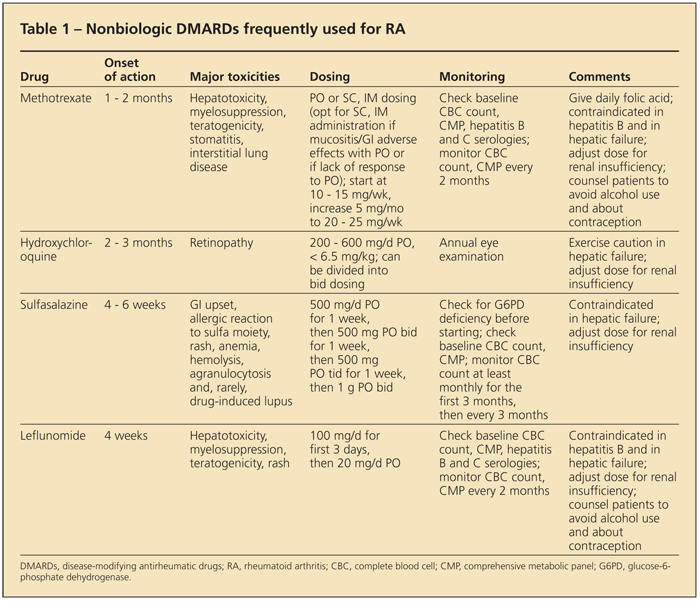
Table 1 summarizes the “nonbiologic” DMARDs that are used frequently. Barring contraindications, methotrexate (MTX) is the DMARD of choice for RA. It suppresses disease activity effectively, and about 30% of patients achieve remission.12 MTX is generally well tolerated, and the dosage can be escalated rapidly.
Subcutaneous or intramuscular administration of MTX can be used in patients who have GI adverse effects or in those who do not respond adequately to oral administration. Because of the potential for hepatotoxicity, MTX should be used only in patients whose serological test results are negative for hepatitis virus, and patients should be counseled to minimize alcohol use. A liver panel and complete blood cell count (to monitor for myelosuppression) should be performed every 2 months.1
As with many DMARDs, MTX is teratogenic; patients should be counseled about contraception as appropriate.13 Interstitial lung disease is another potential complication of MTX. Folic acid should be given along with MTX to reduce the risk of GI and mucosal adverse effects and to prevent hyperhomocysteinemia (an independent risk factor for coronary artery disease).1
Other DMARDs frequently used in RA management are hydroxychloroquine (HCQ), sulfasalazine (SSZ), and leflunomide. Although retinopathy is rare, it is a risk of HCQ treatment; therefore, annual ophthalmological examination is required. GI complaints are common with SSZ; allergic reactions (to the sulfa moiety) and rash also are possible. Leflunomide is another effective DMARD that requires monitoring similar to that needed with MTX.
Other available but rarely used DMARDs include minocycline, azathioprine, cyclosporine, and tacrolimus.1 Gold salts and penicillamine are not used in the United States. A systematic review that compared the effectiveness and harm of DMARDs for RA did not support the use of one over another as monotherapy.14
Adjunctive role for
corticosteroids
Corticosteroids play an important adjunctive role in the management of RA. Systemic corticosteroids may be used to “bridge” to DMARD therapy because the onset of action of DMARDs is delayed (see Table 1). Intra-articular corticosteroid injections are very effective for managing inflammation in a single joint (or a few joints). Randomized controlled data also indicate that corticosteroids reduce the rate of radiographic progression.15 Systemic corticosteroid therapy must be used with caution.13
Targeted biologic therapies
The development of targeted biologic therapies in recent years has greatly affected the treatment of patients with RA (Table 2
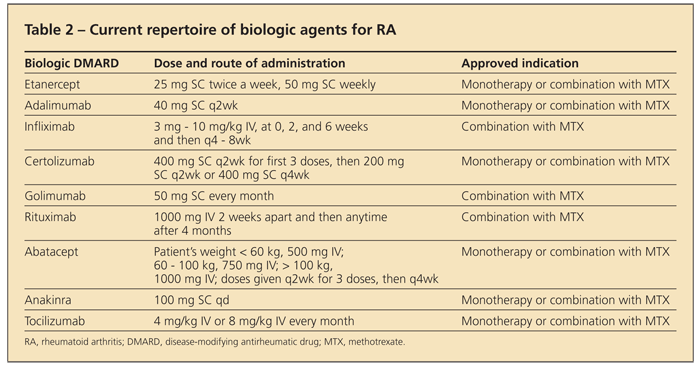
). TNF-α, a proinflammatory cytokine found in high concentrations in the rheumatoid joint, is a major contributor to the inflammatory cascade, resulting in symptoms (eg, pain, stiffness, and joint swelling) as well as joint damage.
The TNF-α–blocking medications that are available and FDA-approved for use in RA are infliximab, etanercept, adalimumab, certolizumab, and golimumab.16 These agents have shown promising results in randomized controlled trials (mostly studied in combination with MTX compared with MTX monotherapy).
An ACR 50 response (the proportion of patients who have a 50% or greater improvement in clinical and laboratory parameters) is a frequently studied outcome. In trials of early RA, ACR 50 responses occurred in a greater number of patients treated with TNF-α inhibitors plus MTX than with MTX alone (odds ratio [OR], 2.17; 95% confidence interval [CI], 1.78 to 2.64; number needed to treat, 6).17 Combination ther-apy also has been shown to have greater efficacy in established RA.18 No well-controlled comparative trials have been performed guiding the initial choice of agent or suggesting efficacy of one agent over another.19
TNF-α inhibitors increase the risk of serious infection (OR, 2.01; 95% CI, 1.31 to 3.09, compared with patients who have RA and are receiving nonbiologic DMARDs).20 Because TNF-α plays a role in the immune response to mycobacterial and fungal infections, new infection with Histoplasma and Coccidioides and reactivation of tuberculosis (TB) are particularly concerning. Patients should be screened for TB before therapy is started, and patients living in or visiting areas in which fungal infections are endemic need to be monitored.
All of the biologic agents are contraindicated in patients with hepatitis B. Caution should be exercised and expert consultation considered before starting the agents in patients who have any chronic infection.
The risk of lymphoma with the use of TNF-α inhibitors is controversial and is confounded by the known increased risk of lymphoma in patients with RA irrespective of their therapy. Moderate to severe congestive heart failure and demyelinating disease are relative contraindications to the use of these drugs. In addition, TNF-α inhibitors should be avoided during pregnancy. A practical consideration is the agents' substantial cost.18
Other effective biologics
Other biologic agents that are effective in RA include rituximab (anti-CD20 chimeric monoclonal antibody), abatacept (cytotoxic T-lymphocyte–associated antigen 4 immunoglobulin construct), and tocilizumab (humanized anti-interleukin [IL]-6 receptor antibody).2 One of these agents should be considered in patients who have moderate to severe RA and have had an inadequate response to at least 1 TNF-α inhibitor or for whom TNF-α blockade is inappropriate. Abatacept may be used even before TNF-α blockade.16
Anakinra, an IL-1 receptor antagonist, has been approved by the FDA for use in RA, although the drug has not been found to be effective enough and is used rarely.16 Clinical trial results suggest that medications targeting the kinases JAK and SYK may have a role in RA management in the future.21
Future role for
aggressive therapy?
There may be a future role for aggressive therapy in patients who have “undifferentiated inflammatory arthritis” even before they fulfill the diagnostic criteria for RA. A recent placebo-controlled randomized trial with MTX demonstrated postponement of the “development” of RA and radiographic joint damage in anti-CCP–positive patients with undifferentiated arthritis.22 In contrast, corticosteroids did not result in a delay of RA development in patients with early inflammatory arthritis.23 Adoption of the new classification criteria may further influence practice in terms of the patients considered for therapy and the timing of therapy.
The role and appropriate timing of combination therapy has been an area of active research. A randomized, controlled, multicenter study compared 4 treatment strategies in early RA: sequential monotherapy, step-up combination therapy, initial combination therapy with a corticosteroid, and initial combination therapy with the anti–TNF-α agent infliximab. After 2 years, all treatment groups achieved similar disease activity, but control was achieved more rapidly in the last 2 groups, with significantly reduced radiographic progression.24
Another question is whether patients who have not responded to MTX therapy would benefit more from the addition of multiple nonbiologic DMARDs or of a biologic DMARD. In the SWEFOT (Swedish Pharmacotherapy) study, the addition of infliximab for patients who had not achieved low disease activity with MTX monotherapy resulted in better clinical outcomes (EULAR and ACR response criteria) than did the addition of SSZ and HCQ (“triple therapy”).25 In the Treatment of Early Aggressive Rheumatoid Arthritis (TEAR) study, however, triple therapy and the addition of
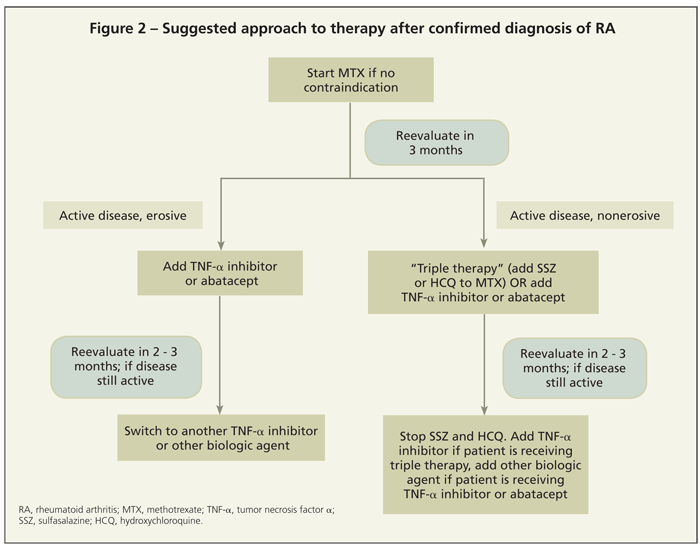
etanercept to MTX were found to be equally efficacious from a clinical standpoint (although follow-up data suggest that the addition of etanercept provides significant radiographic benefit).26,27Figure 2 summarizes a suggested approach to initiating and augmenting therapy.
OTHER ISSUES IN LONG-TERM MANAGEMENT
The potential extra-articular complications of long-standing RA are myriad. They include cardiac disease, such as an increased risk of accelerated atherosclerosis, which accounts for most of the excess mortality in patients with RA. Thus, intensive management of cardiovascular risk factors is required.28
RA also is associated with an increased risk of lymphoma, osteoporosis, skin disease (mainly rheumatoid nodules), ophthalmological disease (including scleritis and episcleritis), and various forms of pulmonary disease.1 However, these complications are being seen much less frequently with the increasing use of newer and more aggressive treatment, although the risk of serious infections is increasing.
Despite the great strides that have been made toward the elusive “cure” RA remains a chronic disease. Its successful management requires a true partnership between the patient and the rheumatologist, as well as close collaboration with the primary care physician.
References:
References
1. Firestein GS, Harris ED Jr, Genovese MC. Rheumatoid arthritis. In: Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley's Textbook of Rheumatology. 8th ed. Philadelphia: WB Saunders Company; 2008:1035, 1097-1104, 1120-1124.
2. Smolen JS, Aletaha D, Koeller M, et al. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861-1874.
3. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315-324.
4. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569-2581.
5. Moreland LW, Russell AS, Paulus HE. Management of rheumatoid arthritis: the historical context. J Rheumatol. 2001;28:1431-1452.
6. van Tuyl LH, Vlad SC, Felson DT, et al. Defining remission in rheumatoid arthritis: results of an initial American College of Rheumatology/European League Against Rheumatism consensus conference. Arthritis Rheum. 2009;61:704-710.
7. Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263-269.
8. Grigor C, Stirling A, Baxter D, et al. 5-year follow-up of the trial of tight control for rheumatoid arthritis (TICORA) study. Arthritis Rheum. 2006;54(suppl 1):S310.
9. Kavanaugh A, Cush JJ. Early, aggressive, and appropriate management of rheumatoid arthritis: focus on benefits and risks of biologic therapies. PeerView in Review 2010. http://www.peerviewpress.com/d/r101. Accessed December 14, 2010.
10. Vliet Vlieland TP. Non-drug care for RA-is the era of evidence-based practice approaching? Rheumatology. 2007;46:1397-1404.
11. Quinn MA, Cox S. The evidence for early intervention. Rheum Dis Clin North Am. 2005;31:575-589.
12. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381-3390.
13. Rindfleisch JA, Muller D. Diagnosis and management of rheumatoid arthritis. Am Fam Physician. 2005;72:1037-1047.
14. Donahue KE, Gartlehner G, Jonas DE, et al. Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann Intern Med. 2008;148:124-134.
15. Kirwan JR. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The Arthritis and Rheumatism Council Low-Dose Glucocorticoid Study Group. N Engl J Med. 1995;333:142-146.
16. Geyer M, Müller-Ladner U. Rationale of using different biological therapies in rheumatoid arthritis. Arthritis Res Ther. 2010;12:214.
17. Ma MH, Kingsley GH, Scott DL. A systemic comparison of combination DMARD therapy and tumour necrosis inhibitor therapy with methotrexate in patients with early rheumatoid arthritis. Rheumatology. 2010;49:91-98.
18. Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006;355:704-712.
19. Which TNF inhibitor for rheumatoid arthritis? Med Lett Drugs Ther. 2010;52:38-39.
20. Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275-2285.
21. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094-1108.
22. van Dongen H, van Aken J, Lard LR, et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007;56:1424-1432.
23. Machold KP, Ladewé R, Smolen JS, et al. The Stop Arthritis Very Early (SAVE) trial, an international multicentre, randomised, double-blind, placebo-controlled trial on glucocorticoids in very early arthritis. Ann Rheum Dis. 2010;69:495-502.
24. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2007;146:406-415.
25. van Vollenhoven RF, Ernestam S, Geborek P, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet. 2009;374:459-466.
26. Moreland LW, O'Dell JR, Paulus H, et al. TEAR: Treatment of Early Aggressive Ra: a randomized, double-blind, 2-year trial comparing immediate triple DMARD versus MTX plus etanercept to step-up from initial MTX monotherapy. Presented at: American College of Rheumatology Annual Scientific Meeting; October 17-21, 2009; Philadelphia. Presentation 1895.
27. Moreland LW, O'Dell JR, Paulus H, et al. Two-year radiographic results from the TEAR trial. Presented at: American College of Rheumatology Annual Scientific Meeting; November 7-11, 2010; Atlanta. Presentation 1368.
28. Hall FC, Dalbeth N. Disease modification and cardiovascular risk reduction: two sides of the same coin? Rheumatology (Oxford). 2005;44:1473-1482.
29. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762-784.




