Article
Mobisante's Mobile Phone Ultrasound Hits US Market
Author(s):
The Redmond, WA–based company Mobisante has released its mobile ultrasound device (MobiUS) nearly nine months after winning FDA 510(K) clearance.
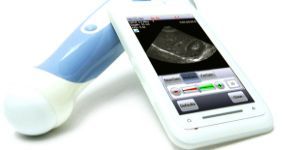
The Redmond, WA—based company Mobisante has released its mobile ultrasound device (MobiUS) nearly nine months after winning FDA 510(K) clearance in February. The delay in availability is largely attributed to the implementation of FDA mandates covering quality control, potential recalls, software updates and product tracking which took the company longer than expected.Earlier this year David Zar, co-founder and CTO of Mobisante confirmed the cost of initial FDA clearance in the low six figures at the West Wireless Health Institute’s HCI-DC event. Despite the high costs, he commented that the extra time has given the company a chance to further refine its product and in the end had been beneficial.
The $7,495 system includes a Toshiba Windows Mobile-powered smartphone, ultrasound probe, and the accompanying Mobisante software. Mobisante’s MobiUS app features eight different types of ultrasound exams with presets appropriate to the individual test. The exams include “Quick Scan”, a general purpose setting, AAA, FAST, Cardiac, OB, Pelvis, Vascular and small organs. Settings are managed through a slide scale for more precise scans. All raw data is stored to the phone and can therefore be post-processed directly. Previous scans are stored under a “Review” tab allowing for further alteration and the addition of markups like arrows and measurements to the image. For a full demonstration of the device, see Mobisante's Quick Overview Video.
The device is FDA approved for ultrasound imaging, analysis and measurement in fetal/OB, abdominal, cardiac, pelvic, pediatric, musculoskeletal and peripheral vessel imaging and makes use of both cellular and WiFi to send images for diagnosis, second opinion, or Picture Archiving and Communication Systems.
The systems delays become evident when considering the included hardware. MobiUS requires USB 2.0 functionality and will therefore only run with the Windows Mobile 6.5-based Toshiba TG01, a smartphone released in 2009. Zar is disappointed the lack of USB 2.0 support by popular devices like Apple’s iPhone and platforms running Google Android OS. Prompted by the interest of potential clients in support for healthcare specific tablets like those available through Panasonic and Motion Computing, Mobisante is now looking into tablet-based solutions for the device.
"These are tablets health care professionals have had at the bedside for years," Zar said. "[MobiUS] would be an added benefit for the platform they already have in place. For many, this is not a replacement system. We can also offer the full imaging solution, though, and that might appeal to smaller clinics. Our work has just begun, really."
Source: Mobisante's Quick Overview: MobiHealthNews





