Publication
Article
Hemophilia Reports
News From EULAR 2012: New Drug Targets Emerge for RA
As basic research on new targets for rheumatoid arthritis (RA) treatment continues, novel therapies move through phase II trials.
A
s basic research on new targets for rheumatoid arthritis (RA) treatment continues, novel therapies move through phase II trials. Multiple approaches to therapy continue to be needed because RA and other related inflammatory diseases continue to progress in most patients despite additions to and switching of therapies. New drugs with different mechanisms of action can halt erosive effects for a time, thereby delaying disease progression.
Recent insights into the processes driving the autoimmune attack underlying RA have led to promising new treatments, many of which were presented at the 2012 European League Against Rheumatism (EULAR) Annual Congress in June.

Many of the “new” targets being tested are only new to these arthritis diseases and have been used in other therapeutic areas, most often in cancer patients. These include granulocyte-macrophage colonystimulating factor (GM-CSF) and transforming growth factor-beta agents, both familiar in the cancer world.
A summary of abstracts presented at the 2012 EULAR Annual Congress are presented here. Selections were chosen because they shed light on several emerging changes in treatment with targeted therapies.
IL-20: A Target for RA Therapy?
Interleukin-20 appears to be a valid drug target in rheumatoid arthritis (RA), according to placebo-controlled trial results with a monoclonal antibody designed to inactivate the protein. However, the trial was small (67 patients), and results were affected by presence of rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA).
In this phase II trial, across all 67 patients, the monoclonal antibody NNC0109-0012 reduced Disease Activity Scale- (DAS) 28 scores including C-reactive protein measurements (DAS28-CRP) by 0.88 points relative to placebo in 12 weeks of treatment. (P = .020).
When the analysis was restricted to the approximately two-thirds of patients in both treatment groups who were positive for RF and ACPA, the mean DAS28-CRP scores declined by about 0.7 points in the placebo group versus 2.4 points with the study drug (P = .0004).
Interleukin-20 (IL-20) is secreted from synovial tissues, which also contain receptors for the cytokine. Studies in animal models of RA indicate that anti-IL-20 monoclonal antibodies relieve pain and inflammation and prevent joint erosion.
In the current trial, treatment was stopped at week 12 (except for methotrexate), but patients were followed and evaluated for an additional 13 weeks. During that time, the difference in responses in sero-positive patients was maintained, with only slight increases in DAS28-CRP scores in both groups from weeks 12 to 25.
Source:
Šenolt L, Göthberg M, Valencia X, et al. Efficacy and safety of NNC0109-0012 (anti-IL-20 Mab) in patients with rheumatoid arthritis: results from a phase 2A trial. Ann Rheum Dis. 2012;71(suppl 3):152. Abstract LB0004.
Stelara Investigated for Use in Psoriatic Arthritis
Study results presented at the 2012 EULAR Annual Congress indicated that ustekinumab (Stelara), an agent already used in psoriasis, may help ease swollen joints in patients with active psoriatic arthritis.
In the phase III PSUMMIT I study, treatment with the interleukin (IL)-12 and IL-23 inhibitor ustekinumab led to 20% improvements in the ACR20 criteria in 42.4% of patients receiving 45 mg and in 49.5% of patients receiving 90 mg, compared with 22.8% of patients treated with a placebo (P < .001).
Psoriatic arthritis—a deforming and debilitating arthritis characterized by inflammation of the synovium, enthesis, and periosteum—develops in approximately 10% to 15% of patients with psoriasis. Current treatment options include conventional disease-modifying drugs such as methotrexate and sulfasalazine, nonsteroidal anti-inflammatory drugs, and biologic TNF inhibitors; however, many patients have inadequate responses to these approaches.
Good results on skin involvement were expected because ustekinumab is already licensed and in clinical use for psoriasis. Researchers said that the real test in this trial was whether the treatment also worked in the joints, and the answer, at least in comparison with placebo, was that it did.
A 75% improvement in skin involvement was observed in 57.2% of patients receiving the 45-mg dosage and in 62.4% of patients receiving the 90-mg dosage, compared with 11% of those receiving placebo.
Source:
McInnes I, Kavanaugh A, Gottlieb AB, et al. Ustekinumab in patients with active psoriatic arthritis: results of the phase 3, multicenter, double-blind, placebo-controlled PSUMMIT 1 study. Ann Rheum Dis. 2012;71(suppl 3):107.
Head-to-Head Drug Trials in RA
Results of the first two head-to-head trials of biologic agents for RA were reported at the EULAR Congress in Berlin, Germany. According to news reports of the congress proceedings, these trials caused significant discussion both on and off the floor of the presentations.
Driven by regulatory requirements in Europe, drugs for RA and related conditions are increasingly being tested against other active agents in clinical trials, but the relevance of the results for clinical practice is a matter of debate, according to EULAR president Maxime Dougados, MD, of René Descartes University in Paris, who is also an active researcher in the field.
With the caveat suggested by Dougados, the two head-to-head trials that follow offer new information that may or may not help in making prescribing decisions concerning choice of biologic agents for RA.
Tocilizumab (Actemra, Genentech) vs Adalimumab (Humira, Abbott) as Monotherapy for RA
Tocilizumab is an infused agent, currently indicated for the treatment of adult patients with moderatelyto- severely-active rheumatoid arthritis who have had an inadequate response to one or more TNF inhibitors. It can be given with or without methotrexate.
Classified as a non-TNF biologic, tocilizumab is an interleukin 6 (IL-6) receptor monoclonal antibody. IL-6 is a pro-inflammatory cytokine produced by a variety of cell types, including T and B cells, lymphocytes, monocytes, and fibroblasts.
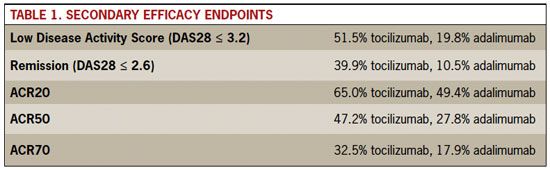
In this randomized, double-blind trial, tocilizumab was significantly more effective than adalimumab as monotherapy for patients with RA who were unresponsive to or unable to take methotrexate. Adverse events were similar between the two agents. In all efficacy measures, tocilizumab was higher than adalimumab.
Table 1. Secondary Efficacy Endpoints
Endpoints
Rollover to enlarge.
Under current guidelines, most biologic drugs are not recommended as monotherapy in the US or Europe. Instead, a combination regimen with methotrexate or other conventional DMARDs is usually preferred. Nevertheless, in practice, a significant number of patients do receive biologics as single agents. Reasons vary: Some patients do not tolerate methotrexate well, or a copay issue might cause some to use a single agent only, or a patient’s preference may favor taking a single drug.
The 24-week trial randomized 326 patients to either 8 mg/kg of tocilizumab by IV infusion every 4 weeks, or 40 mg of adalimumab, by subcutaneous injection every 2 weeks.
On the study’s primary outcome measure, change in Disease Activity Scale-28 (DAS28) score from baseline, tocilizumab was clearly superior, with a decline in mean scores of 3.3 points versus 1.8 with adalimumab (P < .0001). The secondary efficacy endpoints of response rates in each study arm also favored tocilizumab (see Table 1).
Source
Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy is superior to adalimumab monotherapy in reducing disease activity in patients with rheumatoid arthritis: 24-week data from the phase 4 ADACTA trial. Ann Rheum Dis. 2012;71(suppl 3):152. Abstract LB0003.
Abatacept (Orencia, Bristol-Myers Squibb) vs Adalimumab (Humira, Abbott)
The head-to-head trial of these two biologic therapies for RA resulted in a draw, according to many reports from the 2012 EULAR Annual Congress, where the data were presented. Multiple measures of clinical response in patients with early rheumatoid arthritis showed that the two biologic drugs were equally effective.
Abatacept is an anti-CD28 peptide that blocks T-cell activation, and adalimumab is the leading TNF inhibitor. The study’s primary endpoint was efficacy after 1 year, the results of which were reported at the EULAR meeting. The study will continue for another 12 months. Study participants had had an inadequate response or relapse after treatment with methotrexate alone and had not yet received biologic therapy.
Both drugs were given with methotrexate, according to their currently approved labels. Abatacept was given by subcutaneous injection at 125 mg weekly; the adalimumab dosage was 40 mg every other week.
The study’s primary endpoint was 20% reduction in symptoms by American College of Rheumatology criteria (ACR20) at 1 year. Response rates for for abatacept were 64.8%, and 63.4% for adalimumab, meeting the prespecified definition for abatacept noninferiority.
Rates of low disease activity (DAS28 score ≤ 3.2) and remission (DAS28 <2.6) at 1 year were also virtually equal, according to Michael Schiff, MD, the researcher from the University of Colorado in Denver who served as the trial’s lead investigator.
Compared with TNF inhibitors, abatacept has a reputation for relatively slow onset of action, but in the trial, responses over time tracked closely in the two study arms. At week 2, there was a statistically insignificant advantage for adalimumab in ACR20 response rates and DAS28 scores.
Eighty-five percent of patients on abatacept were rated as radiographic nonprogressors versus 89% of the adalimumab group. Rates of severe adverse events and serious infections were similar
Source:
Schiff M, Fleischmann R, Weinblatt M, et al. Abatacept SC versus adalimumab on background methotrexate in RA: one year results from the AMPLE study. Ann Rheum Dis. 2012;71(suppl 3):60. Abstract OP0022.
