Publication
Article
Hemophilia Reports
Rheumatoid Arthritis: Targeted Therapies in the Pipeline
Researchers are testing promising new biologic agents that target different aspects of the inflammatory pathway in rheumatoid arthritis (RA).
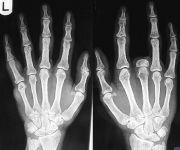
1
The biologic DMARDs either target different B cells and T cells to inhibit their immunologic response or they inhibit TNF, the major proinflammatory cytokine that recruits macrophages and lymphocytes to joints and cause damage in rheumatoid arthritis.2 Newer drugs in phase III clinical trials are targeting different inflammatory mediators, such as interleukin 6 (IL-6), janus kinase 1 and 2 ( JAK1 and JAK2), and granulocyte-macrophage colony-stimulating factor (GM-CSF).2
IL-6 Inhibitors
Sirukumab is a monoclonal immunoglobulin G1 kappa antibody developed by Janssen Biotech Inc.3 The company is investigating sirukumab in phase III trials for patients with RA who failed anti-TNF therapy, while phase II studies focused on patients with RA who did not achieve remission withmethotrexate therapy.3 Sirukumab will be the first in the class of drugs that is inhibits IL-6, a cytokine, which occurs naturally in the body that mediates inflammation and autoimmune conditions.3
IL-6 is being studied because it has been found at increased levels in the blood and synovial tissue in patients with RA. Tocilizumab, an anti-IL-6 receptor blocker, is on the market, but there are no approved medications that directly target IL-6.3 Phase II part A studies showed that at the end of 12 weeks, patients receiving sirukumab therapy—100 mg subcutaneously every 2 weeks—had a greater mean improvement from baseline in Disease Activity Score 28 based on C-reactive protein (DAS28- CRP); statistically significant greater American College of Rheumatology 20% improvement criteria (ACR20) response rate; good/moderate DAS28-CRP response rate; and greater mean improvements in the Health Assessment Questionnaire-Disability Index (HAQ-DI) score and in the Clinical Disease Activity Index (CDAI).3 There was clinical response in patients using sirukumab as early as week 2 of the trial.3
Part B of the study looked at different doses of sirukumab, including 100 mg every 2 weeks, 100 mg every 4 weeks, 50 mg every 4 weeks, and 25 mg every 4 weeks, and placebo with crossover to sirukumab at week 12.3 The group using 100 mg of sirukumab every 2 weeks was the only one to reach the primary end point of American College of Rheumatology 50% improvement criteria (ACR50) at week 12, while other groups did show greater ACR50 response rates that were not statistically significant.3
In the trials, the most common adverse events reported were infections, especially nasopharyngitis and upper respiratory tract infections, elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), hematologic changes (decrease in white blood cell count, neutrophils, platelets), increased lipid levels, and mild injection site reactions.3
The ACR20 and ACR50 response rates seen in the study are similar to those of other anti-IL-6 drugs being studied, tocilizumab, sarilumab, and clazakizumab, in patients with prior methotrexate therapy. The phase II trials show that monoclonal antibodies targeting IL-6 could have efficacy in RA similar to that achieved with IL-6-receptor blockade and TNF inhibition, although there have been no head-tohead trials.3
However, a 68-week study began in 2014 comparing sirukumab monotherapy with adalimumab monotherapy for the treatment of active RA in patients who have had previous treatment failure with methotrexate.4 Along with sirukumab, Alder Biopharmaceuticals and Bristol-Myers Squibb are collaborating in moving clazakizumab into phase III trials.5
For sarilumab, developed by Sanofi and Regeneron, there is promising phase III data as a high-binding affinity monoclonal antibody to the IL-6 receptor to mediate the inflammatory signaling cascade.6 In phase II studies, 306 patients were randomized to receive 100 mg or 150 mg every 2 weeks, 100 mg or 150 mg weekly, 200 mg every 2 weeks, or placebo, with all groups in combination with methotrexate to prove efficacy by improvement of >20% according to ACR20.6 Results of the study showed that 67% and 65% of patients receiving 150 mg and 200 mg every 2 weeks, respectively, achieved ACR20 response at week 12.6
Sarilumab has also shown phase III statistically significant improvements in 3 end points, demonstrating improvement in disease signs and symptoms at 24 weeks, physical function at 16 weeks, and inhibition of joint damage progression at 52 weeks.7 In the phase III SARIL-RA-MOBILITY trial, 1179 patients with active moderate to severe RA who did not respond to methotrexate therapy were randomized to 1 of 3 treatment arms.7 Patients received either 150 mg or 200 mg sarilumab subcutaneously every 2 weeks or placebo, all in combination with methotrexate. Both sarilumab groups showed statistically significant improvement in ACR20 scores at 24 weeks, improvement in physical function at week 16 measured by HAQ-DI, and inhibition of progression of damage measured by modified Total Sharp Score (mTSS).7 The ACR70 response was seen more in patients taking sarilumab and methotrexate versus placebo and methotrexate. 7 The ACR70 scores were 13% in sarilumab 150 mg, 15% in sarilumab 200 mg, and 3% in placebo groups, which showed a statistically significant difference.7 The most common adverse effects reported in the trial were infections, dose-dependent decrease in neutrophil counts, and increase in lowdensity lipoproteins (LDL) and transaminases, all of which were seen more in the sarilumab groups.6
JAK1 and JAK2 modulators
A new effort in developing oral medications for RA began with the approval of tofacitinib (Xeljanz) in 2012.8 Tofacitinib is a JAK inhibitor that prevents the activation of signal transducers and activators of transcription and therefore decreases production of proinflammatory cytokines associated with RA.8 The activation of JAK signals and activates other cytokine receptors and interleukins that cause inflammation; therefore, blocking JAK signaling is a potential new approach for fighting inflammatory diseases. Baricitinib is a selective JAK1 and JAK2 inhibitor created by Eli Lilly and Incyte Corporation that is in phase III studies for treatment of RA.9 In 2013, results from the 52-week phase II part B study were released at the European League Against Rheumatism (EULAR) annual congress, which showed that patients receiving baricitinib therapy had ACR response and low disease activity at week 52 that was sustained from week 24 of the study.9 The study included 301 patients who had >8 swollen and tender joints, with stable use of methotrexate for >12 weeks, who had not received biologic DMARD therapy and were initiated on either 1 mg, 2 mg, 4 mg, or 8 mg daily of baricitinib or placebo. After 24 weeks, patients had either continued or increased their dose to 4 mg or 8 mg daily of baricitinib and results of clinical efficacy from week 24 to week 52 of ACR70, CDAI remission, and DAS28-ESR <3.2 were sustained or improved.10 Patients on baricitinib therapy achieving ACR70 went from 21% to 27% from week 24 to 52, CDAI remission went from 17% to 21%, and DAS28-ESR went from 28% to 42%.10 Study participants with >1 treatment-emerged adverse event (TEAE) stayed consistent from weeks 0 to 24 to weeks 24 to 52.10 In the study, 62% of patients on 4 mg experienced TEAE at 0 to 24 weeks and 53% of patients at 24 to 52 weeks, while 72% of patients on 8 mg experienced TEAE at weeks 0 to 24 and 63% of patients had TEAE at weeks 24 to 52.10 Common incidences reported were infections, anemia, nausea, and dyslipidemia. 10 Barcitinib, as a more specific JAK1 and JAK2 inhibitor, shows that it could open doors for a new oral treatment class for RA.10
Novel approaches targeting GM-CSF
Inhibiting GM-CSF receptor is a novel mode of inhibition of the inflammatory process for the treatment of RA.11 GM-CSF activates neutrophils and macrophages, which promote cytokines and chemokines to synovial tissue, causing inflammation and exacerbation of RA.11 AstraZeneca has developed mavrilimumab as a monoclonal antibody that targets the alpha receptor of GM-CSF to inhibit further activation of macrophages for treatment of moderate to severe RA.11 In a 12-week, phase II study, 233 intent-to-treat patients were separated into treatment arms of subcutaneous 10 mg, 30 mg, 50 mg, 100 mg every other week of mavrilimumab or placebo to reach the primary end point of reduction from baseline of DAS28-CRP of >1.2 points at 12 weeks.11 There was a significant difference in DAS28-CRP from baseline in the treatment groups receiving 50 mg and 100 mg mavrilimumab, and the 100-mg group also had statistically significant improvement in HAQ-DI versus placebo andin treatment effect based on ACR20, ACR50, and ACR70.11 Efficacy of 100-mg group was maintained through follow-up of week 24 with a >1.2 reduction in DAS28-CRP (59%) versus placebo (33%).11 A change in IL-6 and acute phase proteins after 1 month of treatment with mavrilimumab suggests there is suppression of IL-6, which further inhibits the proinflammatory process.11
Adverse events reported in the 24-week study (including follow-up) were 56.9% in patients receiving mavrilimumab and 45.6% in placebo.11 Most commonly seen adverse events were nasopharyngitis, upper respiratory tract infection, and decreased carbon monoxide diffusing capacity because of the decrease in GM-CSF that can affect alveolar macrophage function and surfactant homeostasis in the lungs.11 Mavrilimumab is in phase III trials; however, the phase II trials already indicate acute benefit and sustained effect after treatment termination in patients with RA taking 100 mg every 2 weeks.
References:
1. Rheumatoid arthritis treatment. Johns Hopkins Arthritis Center website. http://www.hopkinsarthritis.org/arthritis-info/rheumatoid- arthritis/ra-treatment/#metho. Accessed July 15, 2014.
2. Burmester GR, Feist E, Dörner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10(2):77- 88.
3. Smolen JS, Weinblatt ME, Sheng S, Zhuang Y, Hsu B. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy [published online 2014]. Ann Rheum Dis.
4. US National Institutes of Health. A study comparing sirukumab (CNTO 136) monotherapy with adalimumab (HUMIRA®) monotherapy in the treatment of active rheumatoid arthritis. Clinicaltrials.gov website. Accessed July 15, 2014.
5. Alder Biopharmaceuticals Inc. Pipeline: current stage of development . http://www.alderbio.com/therapeutics/pipeline/. Accessed July 15, 2014.
6. Huizinga TW, Fleischmann RM, Jasson M, et al. Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARILRA- MOBILITY Part A trial [published online 2013]. Ann Rheum Dis.
7. Regeneron Pharmaceuticals, Inc. Sanofi and Regeneron announce new, detailed data from positive sarilumab phase 3 rheumatoid arthritis trial at EULAR. http://investor.regeneron. com/releasedetail.cfm?ReleaseID=854328. Published June 12, 2014. Accessed July 15, 2014.
8. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2014. 9. Eli Lilly and Company; Incyte Corporation. Lilly and Incyte announce baricitinib efficacy and safety data from the open-label, long-term extension of the phase 2b JADA study in patients with rheumatoid arthritis: results from 52-week study presented at EULAR 2013. https://investor.lilly.com/releasedetail .cfm?releaseid=771109. Published June 13, 2013. Accessed July 15, 2014.
10. Taylor P, Genovese M, Keystone E, et al. Baricitinib, an oral janus kinase inhibitor, in the treatment of rheumatoid arthritis safety and efficacy in an open-label, long-term extension study. Ann Rheum Dis. 2014;73:A31.
11. Burmester GR, Weinblatt ME, McInnes IB, et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann Rheum Dis. 2013;72(9):1445-1452.
Researchers are testing promising new biologic agents that target different aspects of the inflammatory pathway in rheumatoid arthritis (RA). Non-biologic disease-modifying antirheumatic drugs (DMARDs), such as methotrexate, have anti-inflammatory properties, which prevent cytokine production by inhibiting protein synthesis. The resulting increase in adenosine has been shown to have anti-inflammatory effects by binding to the A2 receptor and preventing production of tumor necrosis factor (TNF) and interleukin 8 (IL-8) and interleukin 12 (IL-12), which are all pro-inflammatory mediators that cause an immunologic response.





