News
Article
Oral Povorcitinib for Extensive Nonsegmental Vitiligo Yields Positive Results in Phase 2b Trial
Author(s):
This new data from the phase 2b trial on povorcitinib could lead to developments in the dermatology space for vitiligo patients.
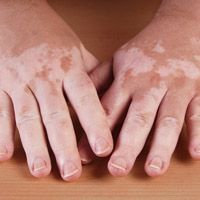
Povorcitinib treatment can lead to substantial total body and facial repigmentation for individuals with extensive nonsegmental vitiligo over all treatment groups, according to new phase 2b trial findings presented at the European Academy of Dermatology and Venereology (EADV) Congress 2023.1
The new 52-week data from EADV was announced by Incyte, and had resulted from a phase 2b clinical trial assessing the safety and the effectiveness of experimental oral JAK1 inhibitor povorcitinib in adults with this particular skin condition.
“Vitiligo often has a significant impact on patients’ lives, and there is a need for new treatment options that can offer solutions to people with extensive disease who desire repigmentation,” Khaled Ezzedine, MD, PhD, Professor at the Université Paris said in a statement. "Today’s results provide an encouraging update in support of a potential oral option to treat extensive nonsegmental vitiligo.”
Ezzedine further commented on his own excitement at observing this continued progress, specifically noting that until recently, this space had limited options for those with nonsegmental vitiligo.
The new data presented at EADV showed that oral povorcitinib treatment expanded upon previously released study results and led to several key findings regarding the drug’s use for vitiligo patients.2
The investigators found that the percentage of improvement in study participants’ total body depigmentation from baseline, as measured by the Total Vitiligo Area Scoring Index (T-VASI) at 52 weeks. It showed values of 40.7%, 42.7%, 41.3%, and 18.1% for groups given povorcitinib 15-to-75 mg, 45 mg, 75 mg, and placebo-to-75 mg groups, respectively.
The percentage of improvement in facial depigmentation from the point of the baseline, looked at by the research team using the facial Vitiligo Area Scoring Index (F-VASI) at 52 weeks, led to values of 63.6%, 63.8%, 64.4%, and 54.8% for the same respective arms of the study.
The investigators also noted that a higher percentage of the on-treatment subjects were able to reach a reduction of ≥50% from the point of baseline in T-VASI (T-VASI50) by the 52-week mark, compared to the findings at 24 weeks.
They added that the percentages were 45.2% for the arm given 15-to-75 mg, 37.0% for the 45 mg arm, 37.9% for the 75 mg arm, and 15.2% for the placebo-to-75 mg arm. Furthermore, more participants who were on-treatment reached ≥50% and >75% reductions from baseline in F-VASI at 52 weeks than at 24 weeks.
Additionally, the investigators wrote that the drug was shown to be well-tolerated at all different dosing regimens. They added that treatment-emergent adverse events of any type or level were shown to have happened in 89.2% of patients who were given 45 mg or 75 mg doses at the time of the study.
The most common ones were noted by the research team, with the list including COVID-19 for 36.1%, greater blood creatine phosphokinase for 13.3%, acne for 12.0%, fatigue for 10.8%, and headache for 9.6%.
“These 52-week results further support earlier data and reinforce the efficacy profile and potential of povorcitinib as an oral treatment for patients with extensive nonsegmental vitiligo,” Kurt Brown, MD, the Vice President and Povorcitinib Global Program Head at Incyte, said in a statement.
The team lastly reported that among the subjects who had done the follow-up through to the 76 week point, subjects’ total body and facial repigmentation were shown to continue to be maintained following the discontinuation of the drug.
This finding indicated a lasting response to povorcitinib, though they acknowledge the value of noting that the post-treatment sample size was fairly small. This may provide a limitation to the investigators’ interpretation of the new data, and additional confirmation using a larger population may be necessary.
References
- Incyte Announces Positive 52-Week Data from Phase 2b Study Evaluating Povorcitinib (INCB54707) in Patients with Extensive Nonsegmental Vitiligo. Incyte. October 11, 2023. Date accessed: October 11, 2023. https://www.businesswire.com/news/home/20231010468675/en/Incyte-Announces-Positive-52-Week-Data-from-Phase-2b-Study-Evaluating-Povorcitinib-INCB54707-in-Patients-with-Extensive-Nonsegmental-Vitiligo.
- Kunzmann K. Oral Povorcitinib Significantly Improves Total, Facial Vitiligo in 24 Week Trial. HCPLive. March 22, 2023. Date accessed: October 11, 2023. https://www.hcplive.com/view/oral-povorcitinib-total-facial-vitiligo-24-week-trial.





