Press Release
Article
Positive Long-Term Data Shown in Patients with Atopic Dermatitis Treated with Rademikibart
Author(s):
These pivotal trial results from a study in China indicated the potential of this drug as a quarterly treatment for individuals with eczema.
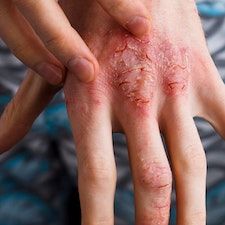
Rademikibart (formerly CBP-201) may be considered safe and effective for individuals with moderate-to-severe atopic dermatitis (AD), according to recent findings.1
This new topline data was announced by Connect Biopharma Holdings Limited, and resulted from a phase 2 trial conducted in China examining the efficacy and safety of rademikibart for patients with AD.
The company announced these new results following prior phase 1 results, in which all primary and key secondary endpoints had been met. The new positive phase 2 results at the 52-week mark demonstrate the potential of the drug as a quarterly treatment for eczema.
Zheng Wei, PhD, the co-Founder and CEO of Connect Biopharma, expressed pleasure at the results of the pivotal trial in China, noting that the improvements seen in participants at 16 weeks in phase 1 were shown to have been maintained with both dosing regimens every 2 weeks and every 4 weeks.
“This study demonstrated that rademikibart has a best-in-class potential, and if approved as a Q4W treatment, we believe could offer patients with AD a highly efficacious treatment with less frequent dosing than current approved treatments,” Wei said in a statement.
The participants featured in phase 2 were responders who had achieved EASI-50 in the 16-week run of the phase 1 trial, and these subjects were randomized to be given rademikibart once every 2 weeks (Q2W) or Q4W. The investigators placed non-responders in an open-label Q2W rademikibart arm.
The research team conducted their analysis of those achieving EASI-75 or IGA 0/1 in both dosing regimens (Q2W and Q4W). The team reported that, at the 52-week mark, 76%-87% were shown to have maintained IGA 0/1 and 92% maintained EASI-75.
The investigators’ assessment of all EASI-50 responders showed sustained improvement from 16 to 52 weeks. They added that 21%-28% more individuals were shown to have reported achievement of IGA 0/1, and 11%-16% more individuals achieved EASI-75.
Additionally, for those getting to a clinically meaningful ≥4-point reduction in peak pruritus numerical rating scale (PP-NRS), the investigators reported that 95.2% were shown to have maintained this level with Q4W dosing and 81.6% with Q2W dosing by the end of their research.
The research team also noted that a ≥5-point reduction on the dermatology life quality index (DLQI) was seen, with 93.4% (Q2W) and 90.0% (Q4W) being shown to maintain this level at the study's conclusion. A 300mg dose at both regimens was also shown to have been fairly well-tolerated with no new safety signals being seen.
References
- Connect Biopharma Announces Positive Long-Term Data from the China Pivotal Trial of Rademikibart in Patients with Moderate-to-Severe Atopic Dermatitis. Connect Biopharma. November 21, 2023. Date accessed: November 22, 2023. https://investors.connectbiopharm.com/news-releases/news-release-details/connect-biopharma-announces-positive-long-term-data-china.





