Publication
Article
MD Magazine Neurology
Promise and Pitfalls of Preventing Migraine With CGRP Inhibitors
Author(s):
The FDA's approval of erenumab marked a milestone in migraine treatment, and although the calcitonin gene-related peptide inhibitor class is full of promise, it has overcome many pitfalls along the way.
Migraine has remained a widely prevalent and disabling disorder despite the efforts of an array of pharmaceutical and nutraceutical agents to prevent or reduce its occurrence. None of the agents were initially developed for the purpose of treating migraines, however, and only a single treatment, onabotulinumtoxinA (Botox, Allergan), was approved for that indication by the US Food and Drug Administration (FDA).
That changed in May with erenumab's approval, and 3 more agents developed to prevent migraine onset by inhibiting a key component in the pathogenesis are now undergoing phase 3 clinical trials (see TABLE). Three of the agents are administered in monthly to quarterly subcutaneous injections and 1 by quarterly intravenous infusion.
The target of each is calcitonin gene-related peptide (CGRP), a neuropeptide that is widely distributed in its alpha isoform throughout the central nervous system and, of particular significance for migraine, within the trigeminovascular system. A ß isoform of CGRP is expressed primarily in the enteric nervous system and involved with inhibiting gastric acid secretion.1
Evidence linking CGRP to migraine includes its elevated level in the external jugular vein during spontaneous migraine attacks and decreasing levels that correspond to symptom relief after acute treatment with a triptan drug product.2 In addition, injection of CGRP can provoke symptoms in migraine sufferers that are indistinguishable from those of spontaneous attacks.3
Finding that CGRP plays a role in migraine led to the development of monoclonal antibodies (MABs) to inhibit its action, with 3 binding to the neuropeptide directly and
1 occupying its receptor.
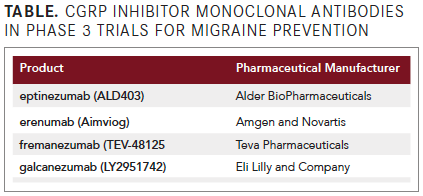
Clinical trials with the 4 investigational CGRP inhibitor MABs are demonstrating reductions of about 3 to 4 migraine days per month in patients with episodic attacks, and about 4 to 6 migraine days per month in patients with chronic migraine who experience ≥15 days per month with headache of any severity and ≥8 migraine days per month.
CGRP Inhibitor MAB Trials
Perhaps more relevant to particular patients than these averaged numbers of reductions is that some patients achieved at least a 75% reduction in their migraine days per month, and others became headache-free throughout the clinical trial period. The efficacy in these patients highlights the importance of further research into the patient characteristics that are relevant to their response and of selecting patients most likely to benefit from the intervention.Pivotal phase 3 trials of erenumab (Aimviog, Amgen and Novartis), which targets the CGRP receptor, and fremanezumab (TEV-48125, Teva Pharmaceuticals), 1 of the 3 that bind directly to the neuropeptide, were published in a recent issue of the New England Journal of Medicine, with an accompanying editorial titled, “CGRP—The Next Frontier of Migraine.”4
In the editorial, Andrew Hershey, MD, PhD, a headache medicine specialist and the Endowed Chair and director of the Division of Neurology at Cincinnati Children’s Hospital Medical Center at the University of Cincinnati College of Medicine, in Ohio, questioned whether the effectiveness of these products will ultimately be determined more from appropriate patient selection than by distinguishing among agents.
“With the ongoing development of 4 different antibodies targeting the CGRP pathway, it will be difficult to determine whether unique patient populations will have a response to a specific drug or whether one agent is superior to others,” Hershey wrote. “Furthermore, many patients will probably still have a response to standard multidisciplinary treatment that is less costly in patient and provider time and dollars.”
The 6-month trial with erenumab involved 955 patients with a history of episodic migraine who reported having between 4 and 15 migraine days per month and fewer than 15 headache days per month on average during the 3 months prior to study screening.5 Patients were randomized 1:1:1 to monthly subcutaneous injections of either 70 or 140 mg erenumab or placebo.
By months 4 to 6, there was a 3.2 mean reduction in migraine days per month in those receiving 70 mg erenumab and a 3.7 mean reduction in days per month in the 140-mg group, compared with a reduction of 1.8 days with placebo. A 50% or greater reduction from individuals’ baseline migraine days per month occurred in 43.3% of those receiving 70 mg and in 50% of those on 140 mg, compared with 26.6% of the placebo group. The adverse events (AEs) with erenumab were reported to be similar to those occurring with placebo.
In the 12-week trial with fremanezumab, 1130 patients with migraine diagnosed as chronic (≥15 days/month) were randomized on a 1:1:1 ratio to receive either quarterly or monthly subcutaneous injections of the drug or placebo.6 The quarterly administered dose was 675 mg, and monthly dosing was 675 mg at baseline and 225 mg at weeks 4 and 8.
The investigators reported a least squares mean (± standard error) reduction in the average number of headache days per month of 4.3 ± 0.3 with fremanezumab quarterly, 4.6 ± 0.3 with monthly administration, and 2.5 ± 0.3 with placebo injections. Abnormalities in hepatic function occurred in 5 patients in each fremanezumab group (1%) and in 3 patients receiving placebo (<1%).
A study of several different doses of galcanezumab (LY2951742, Eli Lilly and Company) recently published in JAMA Neurology was the basis for further trials with 2 doses that were presented at the 2017 American Headache Society (AHS) meeting in Boston, Massachusetts.7 In the multiple-dosage assessment study, 410 patients with migraine history were randomized to receive 3 monthly subcutaneous injections of galcanezumab 5, 50, 120, or 300 mg or placebo. Patients had experienced a frequency of 4 to 14 migraine headache days and at least 2 migraine attacks in the 28-day period prior to screening. The 2 highest doses proved superior to placebo, with the 120-mg dose associated with a reduction from baseline of a median of 4.8 migraine days by the end of the third treatment month, compared with 3.7 with placebo. The frequency of treatment-emergent AEs was similar in drug and placebo.
The subsequent studies (EVOLVE-1 and EVOLVE-2) assessed 120-mg and 240-mg doses of galcanezumab over a 6-month treatment period. In a statement released after presentations at the AHS meeting, the manufacturer indicated that in both studies, 120-mg and 240-mg dosages were statistically significantly superior to placebo in reducing the average number of monthly migraine headaches each month, commencing on the first month of treatment.8 A statistically significantly greater percentage of patients receiving either dose of galcanezumab, compared with those on placebo were reported to achieve at least a 50%, 75%, or 100% reduction in the number of migraine headache days over the 6-month treatment period.
In a January press release announcing results from the PROMISE 2 phase 3 trial with eptinezumab (ALD403, Alder BioPharmaceuticals), the manufacturer indicated that, compared with those receiving placebo, statistically significantly more patients receiving either a single 100-mg or 300-mg intravenous infusion in a 3-month period achieved a 50% or 75% reduction in migraine headaches from baseline.9 Further, 15% of patients receiving eptinezumab had no migraine headaches during the 3-month study. The single quarterly infusion was associated with a mean reduction of 8.2 migraine days per month over the 3-month treatment period, compared with 5.6 days with placebo. AE rates were similar for both drug and placebo, and the statement indicated that the AE profile would be characterized when results of the study are published.
Preventing Potential Pitfalls
“These results represent an important part of the significant step forward that patients who suffer from migraine, many of whom have been living with the disease for decades with limited relief, are about to experience,” Peter Goadsby, MD, PhD, a neurologist and headache specialist at the University of California, San Francisco, Medical Center, said in a January statement.The monoclonal (MAB) antibody CGRP inhibitors have not been associated with the hepatotoxicity that was found with the earlier “gepant” drug class targeting CGRP (see SIDEBAR), possibly because the MABs break down into simpler elements than the metabolic products of the gepant drugs. Hepatic function is among the areas scrutinized in the phase 3 trials, however.
Other concerns include the possibility that inhibition of CGRP could affect a range of systems besides migraine pathogenesis, as CGRP is a ubiquitous peptide with an array of physiological functions. Inhibition of CGRP has the potential to inhibit vasodilation, for example, and there is concern that the agents could possibly inhibit that protective physiological response to cardiac or cerebrovascular ischemia.

Although trials have not identified cardiovascular concerns to date, Goadsby and colleague Amy Tso, MD, a neurologist and headache specialist at the Institute of Psychiatry, Psychology and Neuroscience at King’s College London, in the United Kingdom, pointed out that the incidence of cardiovascular or cerebrovascular disease is low in the studied populations, with a mean age of approximately 40 years.10
“Much larger populations would be needed to see the effect of CGRP blockade on very rare events, and this » would likely only be achieved with postmarketing surveillance. Furthermore, the potential for long-term effects of chronic CGRP inhibition over years even without overt ischemia is entirely unknown,” Goadsby and Tso cautioned.
Another concern is that the MAB agents, although humanized to reduce immunogenicity, still have the capacity to provoke immunologic response. Immunologic AEs have occurred in trials, and antidrug antibodies have been detected, though without evidence in these short-term trials that efficacy is affected.
Given the absence of long-term evidence, Lanfranco Pellesi, MD, from the Medical Toxicology and Headache Center at Policlinico Hospital, at the University of Modena, Reggio Emilia, Italy, and colleagues suggested that cautionary examples can be drawn from chronic administration of biologics in conditions such as rheumatoid arthritis and multiple sclerosis.11 They noted that in each, the emergence of antidrug antibodies has been associated with reduced biological activity and therapeutic efficacy.
“The incidence of patients developing antidrug antibodies, the quantity generated, and their clinical significance are highly variable,” Pellesi and colleagues observed. “This point is highly relevant because the antidrug antibodies may decrease therapy effectiveness and/or facilitate the manifestations of immunoallergic hypersensitivity reactions.”
An additional concern drawn from the biological treatments of other conditions is the anticipated high cost—erenumab, for example, comes in at $575 per month. This factor, along with potentially heightened risk for patients with preexisting ischemic vascular conditions and immunoallergic hypersensitivity, should prompt cost-benefit comparisons with other preventive measures, along with efforts to select patients who are most likely to benefit.12
“An important task will be to attempt to identify patients more likely to benefit so personalized therapy can begin to offset the cost of the treatments by minimizing the number of patients treated who have no useful response,” Tso and Goadsby advised.
This point was emphasized by Brian M. Grosberg, MD, the director of the Hartford HealthCare Headache Center and a professor of neurology at the University of Connecticut School of Medicine, in Farmington, in a discussion with MD Mag.
“If patients have very good efficacy and tolerability on a conventional prophylactic therapy, ie, propranolol or topiramate, and acute treatment, ie, a triptan, then a CGRP inhibitor does not need to be started,” Grosberg said. “However, if their acute and/or preventive treatment regimen is not optimized despite several attempts by a headache specialist, then a CGRP inhibitor should be a consideration.”
Even more resources pertaining to headaches and migraines can be found on MD Magazine's new sister site, NeurologyLive.
REFERENCES
1. Van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21(5):649-678.
doi
: 10.1016/S0149-7634(96)00023-1.
2. Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during
migraine
headache. Ann Neurol. 1990;28(2):183-187.
doi
: 10.1002/ana.410280213.
3. Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with
migraine
with aura. Cephalalgia. 2010;30(10):1179-1186.
doi
: 10.1177/0333102410368444.
4. Hershey AD. CGRP - the next frontier for
migraine
. N Engl J Med. 2017;377(22):2190-2191.
doi
: 10.1056/NEJMe1712559.
5. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of
erenumab
for
episodic
migraine. N Engl J Med. 2017;377(22):2123-2132.
doi
: 10.1056/NEJMoa1705848.
6. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of
chronic
migraine. N Engl J Med. 2017;377(22):2113-2122.
doi
: 10.1056/NEJMoa1709038.
7. Sklijarevski V, Oakes TM, Zhan Q, et al. Effect of different doses of
galcanezumab
vs placebo for episodic migraine prevention: a randomized clinical trial. JAMA Neurol. 2018;75(2):187-193.
doi
: 10.1001/jamaneurol.2017.3859.
8. Lilly’s
galcanezumab
significantly reduces
number
of migraine headache days for patients with
migraine
: new results presented at AHS [news release]. Indianapolis,
IN:
PRNewswire/Eli Lilly and Co; June 10, 2017. prnewswire.com/news-releases/lillys-galcanezumab-significantly-reduces-number-of-migraine-headache-days-for-patients-with-migraine-new-results-presented-at-ahs-300471742.html. Accessed April 10, 2018.
9. Alder announces
eptinezumab
significantly reduces migraine risk meets primary and all key secondary endpoints in pivotal PROMISE 2 phase 3 trial for chronic migraine prevention [news release]. Bothell, WA: Globe Newswire/Alder BioPharmaceuticals Inc. January 8, 2018. globenewswire.com/news-release/2018/01/08/1284947/0/en/Alder-Announces-Eptinezumab-Significantly-Reduces-Migraine-Risk-Meets-Primary-and-All-Key-Secondary-Endpoints-in-Pivotal-PROMISE-2-Phase-3-Trial-for-Chronic-Migraine-Prevention.html. Accessed April 10, 2018.
10. Tso AR, Goadsby PJ. Anti-CGRP monoclonal antibodies: the next era of migraine prevention? Curr Treat Options Neurol. 2017;19(8):27. ncbi.nlm.nih.gov/pmc/articles/PMC5486583. Accessed April 2, 2018.
11.
Pellesi
L, Guerzoni S, Pini LA. Spotlight on anti-CGRP monoclonal antibodies in migraine: the clinical evidence to date. Clin Pharmacol Drug Dev. 2017;6(6):534-547.
doi
: 10.1002/cpdd.345.
12. Lipton RB, Brennan A, Palmer S, et al. Estimating the clinical effectiveness and value-based price range of
erenumab
for the prevention of
migraine
in patients with prior treatment failures: a US societal perspective. J Med Econ. 2018;3:1-26.
doi
: 10.1080/13696998.2018.1457533.






