Article
The Pros and Cons of Ultrasonography for Rheumatologic Conditions
Portable, bedside musculoskeletal ultrasonography (MSUS) equipment has placed the potential for advanced imaging in the hands of office-based clinicians. Assessment of synovial tissue is one of the main applications of MSUS in rheumatology.
ABSTRACT: Portable, bedside musculoskeletal ultrasonography (MSUS) equipment has placed the potential for advanced imaging in the hands of office-based clinicians. Assessment of synovial tissue is one of the main applications of MSUS in rheumatology. On ultrasonography, tendinopathy may manifest as loss of the normal fibrillar structure, fiber heterogeneity, or other findings. MSUS is more sensitive than plain radiography for detecting erosions in some joints of patients with rheumatoid arthritis. It may be a valid method for quantifying cartilage within joint spaces and may be used for investigating median nerve impingement. MSUS has a higher rate of detection of entheseal abnormalities than clinical examination and is more sensitive than other modalities in assessing disease activity. MSUS may guide a number of office procedures, such as aspiration and injection. (J Musculoskel Med. 2011;28:289-295)
Examination of the musculoskeletal system with ultrasonography (US) was first described in the 1950s when Dussik and colleagues1 used it to measure the acoustic properties of articular and periarticular tissue. Interest in musculoskeletal US (MSUS) grew in the 1970s after it was used in animals. As advances in technology have expanded the clinical utility of MSUS, its use has grown significantly, as evidenced by a 157% increase in Medicare beneficiaries from 1996 to 2005.2
The development of higher-frequency transducers allowed for improved resolution of fine structures seen under US and increased sensitivity to detect pathology. The older machines typically were outfitted with single-frequency transducers in the range of 5 Hz to 7.5 Hz. With recent improvements, the machines have transducers that allow for varying frequencies, from 5 Hz to as high as 20 Hz, with axial resolution of 0.038 mm.3
Portable, bedside MSUS equipment has placed the potential for advanced imaging in the hands of clinicians who care for patients in the office setting. Therefore, understanding of its value, pitfalls, and implications becomes increasingly important.
In this article, we review the structures that are studied frequently with MSUS. We also discuss the clinical relevance of MSUS, including comparison with other forms of imaging, and highlight the major advantages and disadvantages of US as it relates to the management of musculoskeletal disease.
BASIC PHYSICS OF DIAGNOSTIC US
The transducer of a US machine produces ultrasonic waves, which are transmitted to the tissues under investigation. These waves are reflected differentially from tissues according to their varying acoustic properties. The reflected waves subsequently are detected by the transducer and translated into an image.
Tissues that reflect ultrasonic waves appear bright or white; tissues that do not reflect ultrasonic waves appear dark or black. Higher-frequency ultrasonic waves generally provide greater resolution of tissue details than lower-frequency waves but do not penetrate tissues as deeply.
STRUCTURES OF IMPORTANCE
Synovial tissue
One of the main applications of MSUS in the field of rheumatology is the assessment of synovial tissue. This includes investigation of synovial effusions, hypertrophy, and hyperemia (Figure 1).
A study that compared MSUS with clinical examination showed that US detects effusions in the knee almost twice as often as does clinical examination.4 Identification of as little as 1 mL of fluid in the hip joint cavity on cadaveric specimens with US has been documented.5 Synovial hyperemia may be detected with power Doppler, which measures the amplitude of motion, in this case blood flow. Doppler signal, highly correlated to the degree of tissue vascularity,6 is a marker for inflammation in synovial tissue. Synovial hypertrophy, defined as hypoechoic intra-articular tissue that is noncompressible, may exhibit Doppler signal.7 MSUS is sensitive to the detection of synovitis in patients with inflammatory arthritis.8
Tendons/ligaments
In a healthy person, tendons are seen as fibrillar structures along their length and as homogeneous oval to round structures in the transverse. On US, tendinopathy may manifest as loss of the normal fibrillar structure, fiber heterogeneity, thickening and effusion of the tenosynovium, calcification, Doppler signal, or a combination of these findings (Figure 2).
FIGURE 1
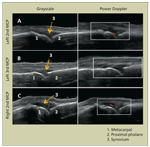
These ultrasonographic images provide a comparison of metacarpophalangeal (MCP) joints in a patient with rheumatoid arthritis. Power Doppler (red signal) is indicative of active inflammation in the left second MCP joint (tender, mildly swollen) (A) and the right second MCP joint (nontender, mildly swollen) (C) but not in the left third MCP joint (nontender, nonswollen) (B).
Resolution to less than 0.2 mm for superficial structures makes MSUS perhaps the most sensitive imaging technique for superficial tendons.3 MSUS also allows the examiner to make dynamic assessments of tendons and ligaments, thus increasing its sensitivity for tendon tears and subluxations.
Bone
Sensitivity for detection of erosive change varies by imaging modality. Radiographs are most often used to determine the existence of erosions. Because it is multiplanar, US is 3 or 4 times more sensitive than plain radiography for detecting erosions in the metacarpophalangeal (MCP) joints and metatarsophalangeal joints of patients with rheumatoid arthritis (RA), especially the second and fifth MCP joints, which are more accessible to US (Figure 3).9-11
Enthesitis may cause the bony attachment points of tendons or ligaments to become irregular or hyperemic or both, an abnormality that MSUS shows well. MSUS also is more sensitive than plain radiography for detecting osteophytes and other bony abnormalities that are associated with osteoarthritis (OA).
Cartilage
MSUS may be a valid and reliable method for quantifying cartilage within the joint spaces of the proximal interphalangeal joints and MCP joints in patients who have inflammatory or noninflammatory arthritis.12 A subtle loss of cartilage contour definition may provide early clues to the development of OA.13
The “double contour sign,” a slightly irregular hyperechoic line over the superficial margin of articular cartilage, is highly specific for gout (Figure 4).14 Its appearance results from the deposition of monosodium urate crystals on the surface of cartilage. In contrast, calcium pyrophosphate dihydrate deposition disease typically manifests as a thin hyperechoic line within the cartilage, correlating well with histological findings in chondrocalcinosis.14
Compressive neuropathies and soft tissue masses
MSUS may be used for investigating median nerve impingement by detecting nerve swelling proximal to the point of compression. This finding has been shown to correlate highly with electromyographic alterations in carpal tunnel syndrome.15 Similarly, entrapment of the ulnar, peroneal, and posterior tibial nerves may be evaluated.
FIGURE 2
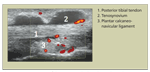
Tenosynovitis of the posterior tibial tendon is shown here. Synovial hypertrophy and effusion surrounding the tendon with Doppler signal (red) indicate inflammation.FIGURE 3
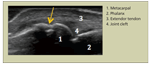
Erosion at the metacarpophalangeal joint (arrow) is seen in a patient with rheumatoid arthritis.FIGURE 4
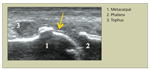
This image shows a metatarsophalangeal joint with a tophus, which has a characteristic speckled appearance, and an anechoic rim. The irregular second bright line over the metatarsal head (arrow) is the “double contour sign” produced by precipitation of monosodium urate crystals on the surface of the hyaline cartilage.
Common soft tissue masses, such as rheumatoid nodules and tophi, may be defined with US. Gouty tophi appear as heterogeneous (hypoechoic and hyperechoic) masses, sometimes with calcifications or an anechoic rim (see Figure 4). Rheumatoid nodules are more homogeneous and hypoechoic masses, possibly with a very hypoechoic central area representing necrosis.16
CLINICAL RELEVANCE OF MSUS
Diagnosis and classification of disease
The history and physical examination remain the cornerstones of clinical evaluation of patients with rheumatologic conditions. However, the field of rheumatology is filled with cases of diagnostic uncertainty, and treatment may be delayed as a result. Recent studies suggest that earlier treatment of patients with RA decreases radiological progression and the extent of joint damage.17
Enthesitis is a hallmark for seronegative spondyloarthropathies, forms of arthritis for which a diagnosis potentially is more difficult because of the absence of specific serological tests. The rate of detection of entheseal abnormalities is higher with MSUS than with clinical examination.18 Multiple studies have found that MSUS may help clinicians make a diagnosis and may have an impact on treatment plans.19-21
Karim and associates20 used MSUS to evaluate 100 rheumatology outpatients. They gained added information from the MSUS assessment that resulted in a change in the site-specific diagnosis based on clinical evaluation, the overall diagnosis, and management plans in 53%, 5%, and 53% of patients, respectively.
Monitoring disease activity
Minimizing disease activity is associated with better functional outcomes in patients with inflammatory arthritis.22 MSUS is more sensitive than other modalities in assessing disease activity; as previously described, positive power Doppler signal in synovial sites has corresponded with histological evidence of vascularity, a marker for inflammation.6
Remission
Treatment with the new biologic agents makes remission in patients with RA a reasonable goal. However, many patients in clinically apparent remission according to established, validated criteria that rheumatologists use clinically and in research have synovial hypertrophy or Doppler signal or both on MSUS.23 These US findings correlate with radiological progression of joint damage.24
The long-term functional consequences of persistent joint inflammation found by MSUS but below the level of clinical detection remain to be determined. However, it is evident that clinical standards for determining remission miss a great degree of subclinical disease.
Interventions under MSUS guidance
MSUS may guide a number of office procedures, such as joint and tendon sheath aspiration and injection, nerve block, injection for nerve entrapment syndromes, cyst aspiration, and synovial biopsy.25 The benefits of MSUS in facilitating these interventions include increased certainty of medication delivery to the desired soft tissue space, increased accuracy of needle placement, decreased procedural pain, and improved efficacy of intra-articular injections compared with blind injections.26
COMPARISON WITH OTHER IMAGING MODALITIES
Plain radiography remains an inexpensive, easily obtained imaging modality for bone pathology, but its use is limited by its relative insensitivity (Table). CT is more sensitive than MRI and US for evaluating bony pathology, such as microfractures, periosteal reaction, and bone erosion, but it is an insensitive test for soft tissues.
TABLE
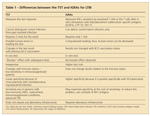
Comparison of musculoskeletal imaging modalities
CT has been considered the gold standard for bone erosion because it is superior to MRI and US. However, studies evaluating CT for this indication have been limited.27 In most joint regions, MRI is better than MSUS for finding erosions.27 Clinicians making decisions about using MSUS or MRI for finding soft tissue abnormalities should consider the structure that is being evaluated and the degree of training that the imaging provider has in MSUS and soft tissue MRI evaluation.
For structures that have limited acoustic windows, such as the anterior cruciate ligaments and meniscal horns in the knees, MRI clearly is the better test. However, a meta-analysis found no difference between US and MRI in sensitivity and specificity for detecting partial- and full-thickness rotator cuff tears.28 The two modalities also perform similarly for other superficial tendon and ligament abnormalities.29,30 In addition, there is a very high correlation between the findings of Doppler US and contrast enhancement on MRI for the detection of superficial tissue inflammation.31
Advantages of US
The use of US in the diagnosis and management of musculoskeletal diseases has many practical advantages (see Table). US is less expensive than other imaging techniques, such as CT and MRI. It does not expose patients to ionizing radiation and does not require contrast for imaging of inflammation, thus avoiding potential problems with contrast-induced allergic reactions; contrast-induced nephropathy; and in the case of gadolinium (pertaining to patients with renal insufficiency), nephrogenic systemic fibrosis. MRI also has the drawbacks of usually requiring at least 30 minutes in an enclosed space, which may not be possible for claustrophobic or obese patients. Each of these factors becomes even more important in the choice of an imaging modality to monitor a patient longitudinally. In one study, 93 of 118 patients preferred US of the shoulder over MRI after undergoing both.32
An obvious advantage of MSUS is that this bedside procedure allows the clinician who is caring for and clinically evaluating the patient to perform diagnostic imaging at the point of care. This effectively diminishes the time between the initial evaluation, diagnostic imaging, and initiation of directed treatment.
Referral of a patient for a musculoskeletal MRI typically would be limited to a complete examination focused on 1 selected joint. The use of US in the office provides the physician with the opportunity to perform focused examinations in multiple regions, including, when necessary, the contralateral side for comparison.
For example, an examiner could perform an enthesitis evaluation of the lower extremities, including the Achilles tendons, plantar fascia, infrapatellar tendons, and quadriceps tendons, for the possible diagnosis of ankylosing spondylitis.33 Or, a fibrocartilage evaluation might include the medial and lateral menisci of the knees and the triangular fibrocartilage of the wrists for the possible diagnosis of calcium pyrophosphate arthropathy. Thus, imaging protocol becomes directed more closely by the clinical suspicion of the physician rather than a task performed by a technician.
Examination with US, unlike with all other imaging modalities, allows for active or passive manipulation of the area under investigation. This may be important in subacromial impingement syndrome and subluxing nerves and tendons.34 In addition, the patient can help direct the examination by indicating the presence or absence of pain during the performance of maneuvers or in response to transducer pressure to correlate with imaging findings. This possibility contrasts with the use of standard MRI, which mandates that the patient be stationary to maintain image quality.
Disadvantages of US
The disadvantages of MSUS include a high degree of operator dependence that can affect the quality of images obtained. This relates to issues of intraobserver and interobserver reliability and the steep learning curve involved in gaining competence in acquiring and interpreting scans.
The escalation in popularity of MSUS has spawned many conferences directed toward educating practitioners in its use. Short courses provide a basis for performing MSUS but are insufficient for participants to become competent without more experience. Inexperienced sonographers can do harm by not performing adequate scans or by misinterpreting US findings or both, especially if they place undue reliance on their findings. Standardization of training, image acquisition technique, and image interpretation methodology can minimize this drawback.
The Ultrasound School of North American Rheumatologists (http://ussonar.org) currently offers rheumatology fellows an 8-month course in MSUS in which standardized techniques for US imaging and interpretation are taught. The Working Group for Musculoskeletal Ultrasound in Rheumatology (a part of the European League Against Rheumatism) has published guidelines on standard scanning.35 A special interest group in the Outcome Measures in Rheumatoid Arthritis Clinical Trials initiative published definitions for pathological findings especially pertinent to inflammatory arthritis.7 However, there remains a deficit of standardized definition of pathology in other areas, such as OA.
To assess interobserver reliability in MSUS, Naredo and associates,36 a group of 23 rheumatologists and 1 radiologist expert in MSUS, performed MSUS on a group of patients independently and blinded to diagnosis. They had overall agreements of 83% and 91% for Doppler signal and joint effusion/synovitis, respectively, for the areas examined (wrist/hand, shoulder, ankle/foot, knee). The investigators represented a select group who had a high level of experience in MSUS; however, similar results can be achieved with examiners who have more limited experience.
In another study, a radiologist expert in MSUS and a rheumatologist with limited experience (50 scans of these areas) used US to examine the hands and feet of patients with RA. They had agreement of 91%, 86%, and 79% in finding erosions, synovitis, and joint effusions, respectively.37
These 2 studies illustrate that consistency can be achieved in performing US. They also demonstrate that acquiring a level of proficiency is not an insurmountable task, although it does require dedication.
Imaging with US involves the transmission, reflection, and capture of sound waves through an acoustic window. Because these sound waves cannot penetrate bones and thick deposits of calcium, structures deep to such tissues (eg, meniscal horns, cruciate ligaments) are hidden from view. In addition, because high-frequency sound waves do not penetrate through deep tissues, musculoskeletal details are shown well at depths greater than 5 cm. This can be a disadvantage for imaging deeply seated joints in obese patients.
SUMMARY
MSUS is a benign, accessible method of evaluating musculoskeletal disease that generally is well tolerated by patients. This technique can allow clinicians to extend their clinical assessment of patients with more objective data without having to rely on the availability of a technician, thus facilitating timelier, more accurate treatment of patients with rheumatologic diseases.
References:
References1. Dussik KT, Fritch DJ, Kyriazidou M, Sear RS. Measurements of articular tissues with ultrasound. Am J Phys Med. 1958;37:160-165.
2. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5:182-188.
3. Grassi W, Filippucci E, Farina A, Cervini C. Sonographic imaging of tendons. Arthritis Rheum. 2000;43:969-976.
4. Kane D, Balint PV, Sturrock RD. Ultrasonography is superior to clinical examination in the detection and localization of knee joint effusion in rheumatoid arthritis. J Rheumatol. 2003;30:966-971.
5. Marchal GJ, Van Holsbeeck MT, Raes M, et al. Transient synovitis of the hip in children: role of US. Radiology. 1987;162:825-828.
6. Walther M, Harms H, Krenn V, et al. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:331-338.
7. Wakefield RJ, Balint PV, Szkudlarek M, et al; OMERACT 7 Special Interest Group. Musculoskeletal ultrasound including definitions for ultrasonographic pathology [published correction appears in J Rheumatol. 2006;33:440]. J Rheumatol. 2005;32:2485-2487.
8. Backhaus M, Kamradt T, Sandrock D, et al. Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 1999;42:1232-1245.
9. Wakefield RJ, Gibbon WW, Conaghan PG, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum. 2000;43:2762-2770.
10. Alarcón GS, Lopez-Ben R, Moreland LW. High-resolution ultrasound for the study of target joints in rheumatoid arthritis. Arthritis Rheum. 2002;46:1969-1970.
11. Lopez-Ben R, Bernreuter WK, Moreland LW, Alarcón GS. Ultrasound detection of bone erosions in rheumatoid arthritis: a comparison to routine radiographs of the hands and feet. Skeletal Radiol. 2004;33:80-84.
12. Hoving JL, Buchbinder R, Hall S, et al. A comparison of magnetic resonance imaging, sonography, and radiography of the hand in patients with early rheumatoid arthritis. J Rheumatol. 2004;31:663-675.
13. Möller B, Bonel H, Rotzetter M, et al. Measuring finger joint cartilage by ultrasound as a promising alternative to conventional radiograph imaging. Arthritis Rheum. 2009;61:435-441.
14. Thiele RG, Schlesinger N. Diagnosis of gout by ultrasound. Rheumatology (Oxford). 2007;46:1116-1121.
15. Visser LH, Smidt MH, Lee ML. High-resolution sonography versus EMG in the diagnosis of carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 2008;79:63-67.
16. Nalbant S, Corominas H, Hsu B, et al. Ultrasonography for assessment of subcutaneous nodules. J Rheumatol. 2003;30:1191-1195.
17. Lard LR, Visser H, Speyer I, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med. 2001;111:446-451.
18. Balint PV, Kane D, Wilson H, et al. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61:905-910.
19. Agrawal S, Bhagat SS, Dasgupta B. Improvement in diagnosis and management of musculoskeletal conditions with one-stop clinic-based ultrasonography. Mod Rheumatol. 2009;19:53-56.
20. Karim Z, Wakefield RJ, Conaghan PG, et al. The impact of ultrasonography on diagnosis and management of patients with musculoskeletal conditions. Arthritis Rheum. 2001;44:2932-2933.
21. Matsos M, Harish S, Zia P, et al. Ultrasound of the hands and feet for rheumatological disorders: influence on clinical diagnostic confidence and patient management. Skeletal Radiol. 2009;38:1049-1054.
22. Wolfe F, Rasker JJ, Boers M, et al. Minimal disease activity, remission, and the long-term outcomes of rheumatoid arthritis. Arthritis Rheum. 2007;57:935-942.
23. Brown AK, Quinn MA, Karim Z, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug–induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54:3761-3773.
24. Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958-2967.
25. Sofka CM, Collins AJ, Adler RS. Use of ultrasonographic guidance in interventional musculoskeletal procedures: a review from a single institution. J Ultrasound Med. 2001;20:21-26.
26. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36:1892-1902.
27. Døhn UM, Ejbjerg BJ, Court-Payen M, et al. Are bone erosions detected by magnetic resonance imaging and ultrasonography true erosions? a comparison with computed tomography in rheumatoid arthritis metacarpophalangeal joints. Arthritis Res Ther. 2006;8:R110.
28. de Jesus JO, Parker L, Frangos AJ, Nazarian LN. Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: a meta-analysis. AJR. 2009;192:1701-1707.
29. Harish S, Kumbhare D, O’Neill J, Popowich T. Comparison of sonography and magnetic resonance imaging for spring ligament abnormalities: preliminary study. J Ultrasound Med. 2008;27:1145-1152.
30. Popovic N, Ferrara MA, Daenen B, et al. Imaging overuse injury of the elbow in professional team handball players: a bilateral comparison using plain films, stress radiography, ultrasound, and magnetic resonance imaging. Int J Sports Med. 2001;22:60-67.
31. Terslev L, Torp-Pedersen S, Savnik A, et al. Doppler ultrasound and magnetic resonance imaging of synovial inflammation of the hand in rheumatoid arthritis: a comparative study. Arthritis Rheum. 2003;48:2434-2441.
32. Middleton WD, Payne WT, Teefey SA, et al. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR. 2004;183:1449-1452.
33. Alcalde M, Acebes J, Cruz M, et al. A sonographic enthesitic index of lower limbs is a valuable tool in the assessment of ankylosing spondylitis. Ann Rheum Dis. 2007;66:1015-1019.
34. Bureau NJ, Beauchamp M, Cardinal E, Brassard P. Dynamic sonography evaluation of shoulder impingement syndrome. AJR. 2006;187:216-220.
35. Backhaus M, Burmester, GR, Gerber T, et al; Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60:641-649.
36. Naredo E, Möller I, Moragues C, et al; EULAR Working Group for Musculoskeletal Ultrasound. Interobserver reliability in musculoskeletal ultrasonography: results from a "Teach the Teachers" rheumatologist course. Ann Rheum Dis. 2006;65:14-19.
37. Szkudlarek M, Court-Payen M, Jacobsen S, et al. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003;48:955-962.

Real-World Study Confirms Similar Efficacy of Guselkumab and IL-17i for PsA



