Publication
Article
Family Practice Recertification
Right-bundle Branch Block and Presyncope in a 25-Year-Old Man
Author(s):
A 25-year-old man with no prior cardiac history presents for evaluation of presyncope. He reports he was shaving when he felt chest pain, shortness of breath and palpitations. He felt dizzy and diaphoretic. Symptoms resolved after 15 minutes.
A 25-year-old man with no prior cardiac history presents for evaluation of presyncope. He reports he was shaving when he felt chest pain, shortness of breath and palpitations. He felt dizzy and diaphoretic. Symptoms resolved after 15 minutes.
He has had one prior episode of syncope resulting in falling down stairs. The fall was a-traumatic and he did not seek medical attention. That syncopal episode was preceded by a feeling of “chest hurting.” He had no drug or alcohol use around the time of either event.
Vital signs and exam were normal. An EKG was performed and is shown below.
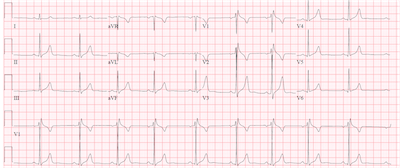
Sinus bradycardia is present with a right bundle branch-like pattern. The QRS is mildly prolonged at 96 ms and there is an R-R’ pattern in leads V1 and V2. There is no slurred wave in the lateral leads that would typically be seen with a right bundle branch block. An echocardiogram was performed showing a structurally normal heart. Because of the ECG pattern, the history of syncope and presyncope with a structurally normal heart, Brugada Syndrome was suspected.
Brugada Syndrome (BrS) is one of the inheritable primary malignant arrhythmia syndromes and can cause recurrent syncope or sudden cardiac arrest (SCA) with polymorphic ventricular tachycardia or ventricular fibrillation (VF).
Brugada Syndrome is seen in 2 per 10,000 people in Western countries and 15 per 10,000 people in Asia and Southeast Asia. It is 8 to 10 times more common in men than women and is passed via autosomal dominant mode of transmission causing mutations in genes. This can affect sodium, calcium or potassium currents. The most common gene affected is the SCN5A gene encoding inward sodium channels, but this mutation is only found in up to 1/3rd or patients with BrS.
Brugada Syndrome can appear for the first time as SCA, but can also appear with syncope, palpitations or chest discomfort, occurring more commonly at night while at rest or sleeping. Arrhythmic episodes are not associated with exercise.
The mean age at presentation is 41 years old and is rarely seen in children. Fever is a precipitating factor and patients with BrS should have fevers treated immediately with antipyretics. There are also a number of provocative drugs that can increase arrhythmic events. A list of inciting drugs in patients at risk, along with other helpful information, can be found on the website brugadadrugs.org.
The diagnosis of BrS has been updated recently and requires a Brugada-pattern on EKG with symptoms. There are 2 predominant patterns. Both have a right bundle branch pattern in V1 and V2, but the slurred S wave in V5 and V6 is notably missing. The first pattern, “coved type”, demonstrates a convex or down-sloping ST segment elevation ≥ 2 mm in ≥ 1 right precordial leads, V1 or V2.
The second pattern is the “saddle back” pattern in which the ST segment descends back to the baseline but remains elevated and then appears to combing into an upright or biphasic T wave. Either pattern may be seen spontaneously or after IV administration of a sodium channel blocking agent when leads are placed in a standard (4th intercostal space) or superior position (2nd or 3rd intercostal space).
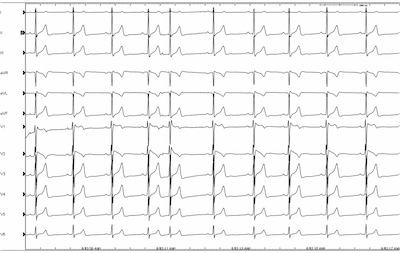
The patient was sent to an electrophysiologist who performed an EP study for definitive diagnosis. An EKG was performed with high right precordial lead placement demonstrating the “saddle back” pattern in lead V1.
Procainamide was given and the “coved type” EKG pattern was demonstrated in lead V2 ultimately confirming the diagnosis of Brugada-pattern EKG.
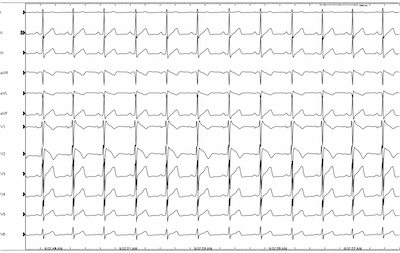
Many patients can have a Brugada-pattern EKG for alternative reasons including atypical right bundle branch block, pulmonary embolism, pericarditis, myocardial infarction and many others. But when faced with syncope in the setting of these EKG findings, BrS must be considered.
In the absence of documentation of VT or VF, other risk factors, including genetic testing, can be used to assess for presence of the syndrome. There are a number of associated conditions with BrS including atrial fibrillation, sleep-disordered breathing and schizophrenia. These conditions have been associated with an increased risk of SCA. For those with concern for a higher risk of SCA, an electrophysiology study to investigate for inducible Brugada-pattern or inducible malignant arrhythmias may be helpful in management.
For anyone with a prior SCA, or sustained VT, an ICD is recommended. A patient with spontaneous Brugada-pattern EKG and history of syncope may benefit from an ICD. Because many people can have inducible VF with provocative maneuvers during an EPS, that alone does not obligate placement of an ICD. After taking into account the patient’s clinical situation and associated factors, ICD may be considered for primary prevention after weighing against potential long-term complications from ICD placement.
Some drugs, like isoproterenol and quinidine, can be used to treat electrical storm in BrS, or to reduce recurrent arrhythmias with ICD shocks. Otherwise there is no proven effective long-term medical therapy. ICD is the only proven effective therapy for BrS.
1. Ackerman MJ, DeSimone CV. Programmed Electrical Stimulation for Patients With Asymptomatic Brugada Syndrome? JACC. 2015;65(9):889—891.
2. Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol 2012; 45:433
3. Naseef A, Behr ER, Batchvarov VN. Electrocardiographic methods for diagnosis and risk stratification in the Brugada syndrome. Journal of the Saudi Heart Association. 2015;27(2):96—108.
4. Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS Expert Consensus Statement on the Diagnosis and Management of Patients with Inherited Primary Arrhythmia Syndromes. 2013;10(12):1932—1963.






