News
Article
Semaglutide: The Drug of Today and a Steppingstone to Tomorrow
Author(s):
An overview of the journey and significant events of 2023 that have catapulted semaglutide from a type 2 diabetes agent to the topic of discussions among circles across healthcare and beyond.
In medicine, a single breakthrough, development, or advancement capturing the attention of clinicians across multiple specialties—let alone the general public—is seldom, if ever, accomplished. But in the year 2023, the world has been captivated by the ascent of semaglutide into the spotlight.
Just 6 years removed from the GLP-1 receptor agonist’s initial approval by the US Food and Drug Administration (FDA) for improving glycemic control in type 2 diabetes (T2D) in 2017, the agent is now positioned at the center of discussions across disciplines in medicine and beyond, as evidenced by the historic demand and ongoing shortage of the agent. Although there has been a demonstrative increase in interest and demand for semaglutide in 2023, the origins of its ascent into the spotlight predates the agent’s 2017 approval by more than a decade.1
In 2005, exenatide (Byetta) became the first GLP-1 receptor agonist to receive approval from the FDA. Between 2005 approval and the 2017 approval of semaglutide, 5 other GLP-1 receptor agonists received approval and came to market, including Novo Nordisk’s liraglutide (Victoza), which received approval in January 2010. Few, if any, could have predicted how the development of liraglutide would set the stage for semaglutide in the next decade and a half.2
Launched in 2010, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial sought to explore the long-term effects of liraglutide on cardiovascular outcomes and other clinically important events. Published in the New England Journal of Medicine (NEJM) in July 2016, results of the LEADER trial, which included 9340 adults with type 2 diabetes, concluded use of the GLP-1 receptor agonist was associated with a significant reduction in the risk of major adverse cardiovascular events, including cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke, compared to placebo.3
Based on the results of Novo Nordisk’s SUSTAIN program, semaglutide 0.5 mg and 1.0 mg (Ozempic) received initial approval from the FDA in 2017 as an adjunct to diet and exercise for improving glycemic control in adults with type 2 diabetes. A clinical program containing 8 trials, the SUSTAIN program would go on to serve as the basis for additional approvals for cardiovascular risk reduction in adults with type 2 diabetes and known cardiovascular disease in 2020 and semaglutide 2.0 mg in 2022.4,5 Although these trials and expanded indications contributed to increased demand for the agent, its use in real-world settings remained limited to patients with type 2 diabetes.
The most significant event leading to the ascent of semaglutide into the conscience of the medical community and general public prior to 2023 occurred with the first release of results from Novo Nordisk’s STEP program, which began in 2021 with the STEP 1 trial. Published in the NEJM in March 2021, the STEP 1 trial randomized adults with obesity or overweight plus 1 weight-related condition who did not have diabetes to semaglutide 2.4 mg or placebo for 68 weeks.
The historic results of the landmark trial—which concluded use was associated with a mean 14.9% reduction in body weight with semaglutide 2.4 mg compared to 2.4% with placebo therapy—sent the interest level in semaglutide 2.4 mg skyrocketing. With data from STEP 1 and other trials within the clinical program providing evidence of the safety and efficacy of semaglutide 2.4 mg (Wegovy), the FDA approved the agent as adjunct to diet and exercise for chronic weight management in adults with obesity or overweight with ≥1 weight-related condition in June 2021.
Since approval for chronic weight management, much of the conversation has centered around issues with access and coverage. It would take less than a year after its approval for chronic weight management for the increased demand for the agent, coupled with the impact of the COVID-19 pandemic, to result in a shortage of semaglutide 2.4 mg. As of November 2023—more than 18 months after the shortage began—the increased demand for semaglutide 2.4 mg outpaced production and, despite additional investment in manufacturing capabilities, there was no foreseeable end to shortage in the future.7
In 2023, the community received news regarding semaglutide, confirming what many had theorized since the LEADER trial and providing new insight into the potential role of GLP-1 receptor agonist therapy for years to come.
The Drug of Today
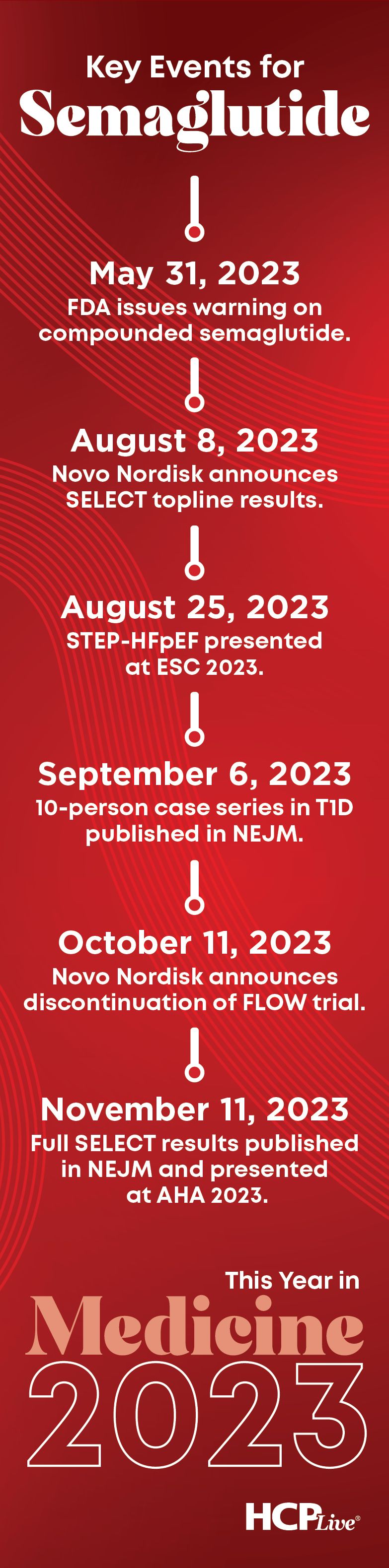
Throughout the second half of 2023, semaglutide was a staple in medical news headlines. In less than 6 months, the community learned of results from a multitude of trials and studies examining semaglutide in different patient populations, ranging from heart failure to chronic kidney disease to type 1 diabetes and—in its crowning moment this year—people with overweight and obesity with preexisting cardiovascular disease.
Prior to the second half of the year, most coverage of semaglutide focused on the ongoing shortage and, subsequently, the warnings from the FDA and Novo Nordisk about the dangers of using compounded versions of the agent, which began to rise in popularity as the shortage continued.8 From this point forward, a consistent flow of news related to the development and potential role of semaglutide would hold the attention of the medical community, beginning with announcement of a topline results from the SELECT trial in early August.9
Touting data evidence of a 20% reduction in risk of cardiovascular death, non-fatal heart attack or non-fatal stroke relative to placebo, topline results offered enough evidence to grip the attention of the medical community until full release of the SELECT trial at a medical conference later in the year.9 Until then, clinicians with piqued interest turned to the STEP-HFpEF trial, which was scheduled to be presented less than 3 weeks later at the European Society of Cardiology (ESC) 2023 Annual Congress.
A randomized, double-blind, placebo-controlled trial conducted at 96 sites in 13 countries in Asia, Europe, and North and South America, the Effect of Semaglutide 2.4 mg Once Weekly on Function and Symptoms in Subjects with Obesity-related Heart Failure with Preserved Ejection Fraction (STEP-HFpEF) trial was designed to explore whether semaglutide 2.4 mg could improve symptoms and physical function among patients with HFpEF and obesity. The trial had dual primary endpoints defined as change from baseline in the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CSS) and change in body weight.10
The trial, which included 529 patients, concluded use of semaglutide was associated with larger reductions in symptoms and physical limitations as well as greater improvements in exercise function and greater weight loss than placebo.10 At the Heart Failure Society of America (HFSA) 2023 Annual Scientific Meeting, trial investigators brought forth additional data fortifying the excitement surrounding the agent’s purported cardiovascular benefit, with results from a prespecified analysis demonstrating benefits of semaglutide observed in the trial were consistent across the spectrum of ejection fraction.11
“We now have a therapy that was tested in a very modest size clinical trial, with STEP-HFpEF having just over 500 patients, but really produced the largest, to my knowledge, quality of life improvement that we've ever seen in a heart failure trial,” Stephen Greene, MD, a heart failure cardiologist with Duke University Medical Center, said in an interview with HCPLive.12
Less than 2 weeks after presentation of STEP-HFpEF, semaglutide was in the headlines again, but this time for a report published in the NEJM detailing its potential benefit among patients with newly diagnosed type 1 diabetes. A 10-person case series from the State University of New York at Buffalo provided an overview of the effects of semaglutide use in people with newly diagnosed type 1 diabetes, with results suggesting use was associated with a complete elimination of the need for mealtime insulin doses among the entire study cohort, allowed 70% to eliminate basal insulin use within 6 months, and decreased the mean HbA1c from 11.7% at baseline to 5.9% at 6 months and 5.7% at 12 months.13 Just 5 weeks after the report was published, Novo Nordisk released an announcement from their ongoing development of semaglutide.14
The ascent of semaglutide into the cardiometabolic spotlight for its potential role in treatment algorithms beyond type 2 diabetes is not unlike that of the SGLT2 inhibitor class in years prior. Like the SGLT2 inhibitor class, many in medicine have their attention turned to the potential for renoprotective benefits of semaglutide use after Novo Nordisk announced they would be halting the FLOW trial in October 2023.14
A randomized, double-blind, parallel-group, placebo-controlled, superiority trial comparing semaglutide 1.0 mg against placebo therapy as an adjunct to standard of care on kidney outcomes in people with type 2 diabetes and chronic kidney disease, the decision to discontinue the trial was based on a recommendation from the trial’s independent Data Monitoring Committee indicating an interim analysis of the kidney outcomes trial for weekly semaglutide had met certain prespecified criteria for stopping the trial early for efficacy.14
“Right now, we have 3 pillars but there is potentially a fourth pillar,” George Bakris, MD, professor of medicine and director of the American Society of Hypertension's Comprehensive Hypertension Center at the University of Chicago Medicine, told HCPLive in September. “We don't know yet, but this could be the GLP-1 RAs and semaglutide. If [FLOW] is positive, then there will be 4 pillars of therapy [for chronic kidney disease].”
While the aforementioned news and data held the attention of specialists within the medical community, the ability of semaglutide to capture the public consciousness was never more apparent than with the presentation and simultaneous publication of results of the SELECT trial on November 11, 2023.
Initiated in 2018, the Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity (SELECT) Trial was launched with the intent of comparing semaglutide 2.4 mg against placebo as an adjunct to cardiovascular standard of care for reducing the risk of major adverse cardiovascular events in people with established cardiovascular disease with overweight or obesity and no prior history of diabetes. Presented at the American Heart Association 2023 Scientific Sessions and simultaneously published in the NEJM, the double-blind, randomized, placebo-controlled, event-driven superiority trial enrolled 17,604 patients aged 45 years or older with a BMI of 27 kg/m2 or greater from more than 800 centers in 41 countries.15
In addition to the aforementioned 20% reduction in risk for the primary outcome, secondary endpoint analyses revealed nonsignificant relative risk reductions of 15%, 18%, 19%, and 73% for cardiovascular death, heart failure hospitalization, all-cause mortality, and progression to type 2 diabetes, respectively. A prominent topic of discussion and source of excitement surrounding the benefit achieved with semaglutide 2.4 mg among this patient population came upon realization of the early separation of event curves observed in the trial.15
“One of the important findings was the survival curves. The differences in event rates in the primary endpoint began to diverge very early—pretty much as soon as you could see it—within a few months after initiation of therapy, which is not what you would expect if the effect was purely from the magnitude of weight loss. It took us an average 65 weeks to reach maximum weight loss and then plateau thereafter,” A. Michael Lincoff, MD, vice chair of research in the Department of Cardiovascular Medicine at the Cleveland Clinic and cochair of the steering committee for the SELECT trial, said in an interview with HCPLive. “So, it clearly is not exclusively linked to the magnitude of weight loss.”
Based on the results, Novo Nordisk filed a supplemental New Drug Application (NDA) for a label update of semaglutide 2.4 mg in the US and Europe to include an indication for reducing the risk of major adverse cardiovascular events. The company expects a decision on this application in 2024.16
At a time when 41.9% of the US population has obesity and cardiovascular disease accounting for 1 in every 5 deaths, the downstream implications of expanded insurance coverage and establishment of obesity as a modifiable risk factor could allow SELECT to stand as one of the most important events in public health since the turn of the century.17,18
“I would argue it's probably one of the most important trials not only of the year, perhaps even the decade because it's going to usher in a new generation of obesity therapies and also evidence generation around obesity,” explained Muthiah Vaduganathan, MD, MPH, codirector of the Center for Cardiometabolic Implementation science at Brigham and Women’s Hospital, in an episode of Don’t Miss a Beat. “The last decade was dominated by diabetes and cardiovascular outcomes trials in this cardiovascular-kidney-metabolic space and we have really developed a very strong foundational evidence base for how to manage high risk people with diabetes. In this decade, we will now see a new era of clinical trials, specific cardiovascular outcome trials, that will focus on people who are obese or overweight and SELECT was really the first of those trials.”
A Steppingstone to Tomorrow
As Vaduganathan described, many feel the time semaglutide has earned in the spotlight could be short-lived relative to its paradigm-shifting impact on public health. As 2023 ends, it is evident the next generation of incretin therapies is well into development, including tirzepatide, a dual GIP/GLP-1 receptor agonist, which is already approved for type 2 diabetes and obesity, as well as a slew of other dual and triple agonist therapies in the pipeline.19
The impending tidal wave of incretin therapies was on display at scientific meetings throughout 2023, but never more apparent than at the American Diabetes Association (ADA) 2023 Scientific Sessions, where the community received formal introductions to a multitude of these incretin therapies in the form of positive phase 2 data from clinical programs for orforglipron, cagrisema, survodutide, and retatrutide.
A nonpeptide oral GLP-1 receptor agonist, orforglipron data provided insight into a pair of phase 2 trials among patients with overweight or obesity and those with type 2 diabetes, respectively. In the weight management trial, use was associated with a mean reduction in body weight up to 14.7% at 36 weeks. In the type 2 diabetes trial, use was mean reduction in HbA1c with up to 2.1% at 26 weeks.20
A coadministered formulation of cagrilinitide and semaglutide, cagrisema was compared against semaglutide or cagrilinitide alone in patients with type 2 diabetes and BMI greater than 27 kg/m2 for 32 weeks. In the trial, a greater proportion patients achieved target HbA1c goals of less than 7.0% and 6.5% or less with cagrisema than with semaglutide or cagrilinitide alone.21
A dual glucagon/GLP-1 receptor agonist, survodutide was examined in a 46-week trial among patients with overweight or obesity without type 2 diabetes. In this trial, use was associated with a mean body weight reduction of 14.9%, with more than 65% of those receiving the 4.8 mg dose experiencing body weight reductions of 15% or greater.22
The next-generation incretin therapy garnering the most attention in 2023, phase 2 data from a pair of trials introduced retatrutide, a GIP/glucagon/GLP-1 receptor agonist, to the medical community. In the weight management trial, which enrolled patients with overweight and obesity without type 2 diabetes, use of retatrutide was associated with a mean body weight reduction of 17.5% at 24 weeks and 24.2% at the end of the 48-week treatment duration. In the type 2 diabetes trial, which enrolled adult patients with type 2 diabetes and BMI of 25 to 50 kg/m2, use was associated with a reduction in mean HbA1c of –2.02% at 24 weeks and no reports of severe hypoglycemia.23 A signal of the potential of dual and triple agonist therapies, further data from the phase 2 trial of retatrutide in patients with obesity presented at the American Association for the Study of Liver Diseases annual meeting. In this analysis, results suggested use was associated with resolution of liver fat in more than 85% of patients.24
Regardless of the potential of therapies in the pipeline or what it may mean for the role of semaglutide decades from now relative to these agents, the world of incretin therapies has staked claim to 2023 as the year of the incretin therapy, with semaglutide sitting atop this evolving landscape.
“I’m excited, I think that we are we are opening a new door to management for lots of people and there's so many more incretins that are coming, with new combinations and new compounds,” explained Natalie Bellini, DNP, in an episode of Diabetes Dialogue. “We're going to be able to offer more and more in the next handful of years with other options and I'm excited about that.”
References:
- Novo Nordisk. Ozempic® (semaglutide) approved in the US. Novo Nordisk. December 5, 2017. Accessed December 5, 2023. https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=712.
- Biopharma PEG. Evolution of GLP‐1 receptor agonists for diabetes treatment. Biopharma PEG. September 22, 2022. Accessed December 5, 2023. https://www.biochempeg.com/article/299.html.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311-322. doi:10.1056/NEJMoa1603827
- Medicine Matters. A quick guide to the Sustain trials. diabetes.medicinematters.com. April 20, 2023. Accessed December 5, 2023. https://diabetes.medicinematters.com/semaglutide/type-2-diabetes/a-quick-guide-to-the-sustain-trials/12206922.
- Drugs.com. Ozempic (SEMAGLUTIDE) FDA approval history. Drugs.com. Accessed December 5, 2023. https://www.drugs.com/history/ozempic.html.
- Wilding JPH, Batterham RL, Calanna S, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Novo Nordisk. Supply update. Novo Nordisk. November 10, 2023. Accessed December 5, 2023. https://www.novonordisk-us.com/supply-update.html.
- Campbell P. SEMAGLUTIDE shortage prompts FDA warning to patients, providers. HCP Live. June 7, 2023. Accessed December 5, 2023. https://www.hcplive.com/view/semaglutide-shortage-prompts-fda-warning-to-patients-providers.
- Campbell P. Select trial shows semaglutide 2.4 mg could reduce cardiovascular risk. HCP Live. August 8, 2023. Accessed December 5, 2023. https://www.hcplive.com/view/select-trial-shows-semaglutide-2-4-mg-could-reduce-cardiovascular-risk.
- Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N Engl J Med. 2023;389(12):1069-1084. doi:10.1056/NEJMoa2306963
- Campbell P. SEMAGLUTIDE provides consistent benefit across ejection fraction in HFPEF. HCP Live. October 8, 2023. Accessed December 5, 2023. https://www.hcplive.com/view/semaglutide-provides-consistent-benefit-across-ejection-fraction-in-hfpef.
- Campbell P. Experts’ perspectives: Top story in heart failure for 2023. HCP Live. October 11, 2023. Accessed December 5, 2023. https://www.hcplive.com/view/experts-perspectives-top-story-in-heart-failure-for-2023.
- Dandona P, Chaudhuri A, Ghanim H. Semaglutide in Early Type 1 Diabetes. N Engl J Med. 2023;389(10):958-959. doi:10.1056/NEJMc2302677
- Novo Nordisk. Novo Nordisk will stop the once-weekly injectable semaglutide kidney outcomes trial, FLOW, based on interim analysis. Novo Nordisk. October 10, 2023. Accessed December 5, 2023. https://www.novonordisk.com/news-and-media/news-and-ir-materials/news-details.html?id=166327.
- Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N Engl J Med. Published online November 11, 2023. doi:10.1056/NEJMoa2307563
- Novo Nordisk. Wegovy® (semaglutide) injection 2.4 mg cardiovascular outcomes data presented at the American Heart Association Scientific Sessions and simultaneously published in the New England Journal of Medicine. Novo Nordisk. November 11, 2023. Accessed December 5, 2023. https://www.novonordisk-us.com/media/news-archive/news-details.html?id=166344.
- Centers for Disease Control and Prevention. Adult obesity facts. Centers for Disease Control and Prevention. May 17, 2022. Accessed December 5, 2023. https://www.cdc.gov/obesity/data/adult.html.
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association [published correction appears in Circulation. 2023 Feb 21;147(8):e622] [published correction appears in Circulation. 2023 Jul 25;148(4):e4]. Circulation. 2023;147(8):e93-e621. doi:10.1161/CIR.0000000000001123
- Eli Lilly and Company. FDA approves Lilly’s zepboundTM (tirzepatide) for Chronic Weight Management, a powerful new option for the treatment of obesity or overweight with weight-related medical problems. Eli Lilly and Company. November 8, 2023. Accessed December 5, 2023. https://investor.lilly.com/news-releases/news-release-details/fda-approves-lillys-zepboundtm-tirzepatide-chronic-weight.
- Campbell P. Orforglipron shows promise as weight loss, diabetes agent in phase 2 trials. HCP Live. June 24, 2023. Accessed December 5, 2023. https://www.hcplive.com/view/orforglipron-shows-promise-as-weight-loss-diabetes-agent-in-phase-2-trials.
- Frias JP, Deenadayalan S, Erichsen L, et al. Efficacy and safety of co-administered once-weekly cagrilintide 2·4 mg with once-weekly semaglutide 2·4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet. 2023;402(10403):720-730. doi:10.1016/S0140-6736(23)01163-7
- Campbell P. Survodutide, formerly Bi 456906, impresses in phase 2 dose-finding trial. HCP Live. June 23, 2023. Accessed December 5, 2023. https://www.hcplive.com/view/ada-2023-survodutide-impresses-in-phase-2-dose-finding-trial.
- Campbell P. Retatrutide showcases historic weight-lowering, glycemic control benefits in phase 2 data. HCP Live. June 28, 2023. Accessed December 5, 2023. https://www.hcplive.com/view/retatrutide-showcases-weight-lowering-glycemic-control-benefits-in-phase-2-data.
- Kunzmann K. Retatrutide clears fatty liver disease in >85% of patients with obesity. HCP Live. November 13, 2023. Accessed December 5, 2023. https://www.hcplive.com/view/retatrutide-clears-fatty-liver-disease-patients-obesity.




