Article
Strategies for Preventive Care in Systemic Lupus Erythematosus
ABSTRACT: Increased disease recognition and therapeutic advanceshave led to improved survival in patients with systemic lupus erythematosus(SLE) over the past several decades. As a result, managementof the long-term comorbidities and complications of SLE has taken ongreater importance. Maintaining a high index of suspicion for cardiovasculardisease in SLE and screening for traditional risk factors isprudent. Minimizing the use of immunosuppressive agents remainsthe main strategy for decreasing infections, but providing routinevaccinations also can decrease the burden of infections. Despite thehigh prevalence of osteoporosis, screening and treatment remainsuboptimal in patients with SLE and deserve increased attention.Patients with SLE are at increased risk for malignancy and physiciansshould remain vigilant for cancer in these patients. (J MusculoskelMed. 2008;25:316-320)
ABSTRACT: Increased disease recognition and therapeutic advances have led to improved survival in patients with systemic lupus erythematosus (SLE) over the past several decades. As a result, management of the long-term comorbidities and complications of SLE has taken on greater importance. Maintaining a high index of suspicion for cardiovascular disease in SLE and screening for traditional risk factors is prudent. Minimizing the use of immunosuppressive agents remains the main strategy for decreasing infections, but providing routine vaccinations also can decrease the burden of infections. Despite the high prevalence of osteoporosis, screening and treatment remain suboptimal in patients with SLE and deserve increased attention. Patients with SLE are at increased risk for malignancy and physicians should remain vigilant for cancer in these patients. (J Musculoskel Med. 2008;25:316-320)
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by frequent disease exacerbations and an unpredictable clinical course. Over the past several decades, increased recognition and awareness of SLE and therapeutic advances-resulting in earlier diagnosis and more aggressive treatment-have dramatically improved survival. Now that many patients with SLE are living longer, however, management of long-term comorbidities and complications has taken on greater importance.
Four areas of SLE comorbidity have gained increased attention: cardiovascular disease (CVD), infection, bone loss, and malignancy (Table). Each complication is increased in SLE and may cause significant morbidity or mortality.
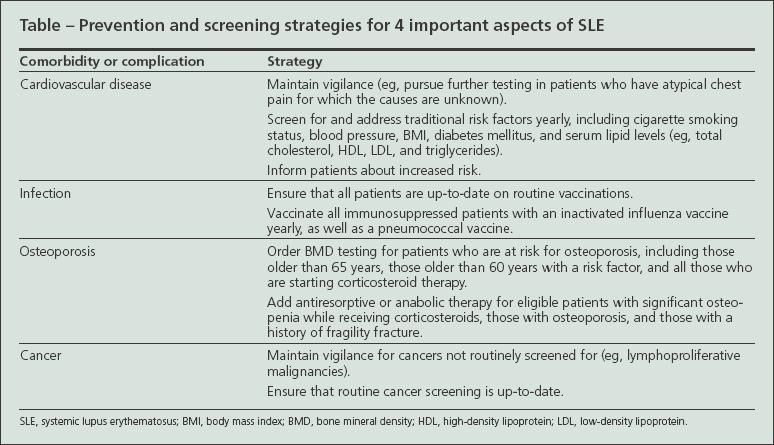
Improving outcomes in these areas will require a stronger emphasis on coordinated interdisciplinary care in SLE. Both the SLE specialist, often a rheumatologist or nephrologist, and the generalist physician need to remain attentive to long-term complications and formalize efforts to coordinate care. Such care coordination probably will require innovations that vary with the practice setting. Electronic medical records, patient reminders, and direct communication via telephone, e-mail, or correspondence among providers provide ways to ensure that the care will occur in a timely fashion.
In this article, I describe the comorbidities and complications of SLE. I also discuss ways to ensure that these conditions are managed in a timely fashion.
COMORBIDITIES AND COMPLICATIONS
Cardiovascular disease: Suspicion and screening
The association between CVD and SLE first became evident almost 4 decades ago, when researchers described a bimodal pattern of mortality in SLE: early mortality resulting from disease-related complications and late mortality resulting from CVD.1 Women with SLE aged 35 to 44 years were 50 times more likely to have a myocardial infarction (MI) than women of similar age in the Framingham Offspring Study,2 a longitudinal cohort study, and women aged 45 to 64 years were about 2 to 4 times more likely to have an MI.3 The risk of other CV events also appears to be elevated in SLE. One study found not only a 10-fold increase in the risk of nonfatal MI in SLE but also striking differences in the risk of death resulting from CVD (relative risk [RR], 17) and stroke (RR, 7.9).4
Several studies have cited an increased prevalence of traditional CV risk factors in SLE compared with controls. Of note, these risk factors do not fully explain the morbidity and mortality of atherosclerosis in this population.4 Research indicates that a number of novel risk factors also may contribute to CVD in SLE.5
The optimal strategy for decreasing CV risk in SLE is still unclear, and research in this area is ongoing. Until such information is available, maintaining a high index of suspicion for CVD and screening for and addressing traditional CV risk factors is prudent.
Maintaining a high index of suspicion. The presentation of ischemic heart disease in SLE can vary greatly; many patients have atypical chest pain, epigastric pain, or pleuristic chest pain, and some patients have no symptoms. In addition, CV events may occur in demographic groups in which the likelihood of disease in the general population is quite low, such as young women.
Therefore, physicians should stay attuned to the epidemiology of CVD in SLE and aggressively pursue a diagnostic workup for any worrisome symptoms or signs. Also, it makes sense to maintain a low threshold for obtaining additional tests (eg, stress imaging studies) and, when questions remain, consider referral to a cardiologist.
Screening for traditional risk factors. Although it is becoming increasingly clear in the literature that traditional Framingham risk factors cannot entirely explain the burden of CVD in SLE, such risk factors appear to play some role in accelerated atherogenesis. Many experts have advocated that physicians think of SLE as a CV risk equivalent, such as diabetes mellitus (DM), and aggressively modify risk factors accordingly. However, clinical trial evidence is needed to prove the efficacy and safety of this approach.
What should physicians do in the meantime? At a minimum, they should screen all adult patients who have SLE annually for traditional CV risk factors (eg, cigarette smoking, DM, hypertension, obesity, and hyperlipidemia). Lifestyle modifications should be recommended (eg, weight loss, exercise, cessation of smoking, and maintaining a healthful diet). Using guidelines for the general population, aggressive management of blood pressure and DM should be instituted and therapy with aspirin should be considered.
The optimal treatment strategy for hyperlipidemia remains under investigation. Until more information is available, a reasonable approach is to follow guidelines for the general population in most patients but consider more aggressive lipid lowering (eg, low-density lipoprotein level lower than 100 mg/dL) for patients with previous vascular events; subclinical CVD on imaging studies; or multiple risk factors,such as the constellation of factors that define the metabolic syndrome.
Informing patients about risks. All patients with SLE should receive counseling about the heightened risk of atherosclerosis. A recent study demonstrated that most patients with SLE have not received CVD risk counseling from their physician,6 suggesting room for improvement in this area.
Preventing infections: The role of immunization
Infections continue to cause significant morbidity and mortality in patients with SLE, particularly those who are receiving immunosuppressive therapy.7 Although minimizing the use of immunosuppressive agents remains the main strategy for decreasing infections in SLE, vaccination may help, especially in immunosuppressed patients.
Vaccination rates in populations with rheumatologic diseases are low.8,9 In SLE, the low rates may partly reflect long-standing concerns about vaccine safety that arose after early reports of fatalities or increased disease activity after immunization. However, several decades of literature have failed to corroborate these concerns. Most studies show no significant increase in disease activity after administration of inactivated vaccines, and few have reported serious adverse events in patients with SLE.
Most experts now agree that patients with SLE should receive the inactivated vaccinations that are recommended for the general population. However, the use of live vaccines remains controversial because of the theoretical risk of vaccine-induced disease in immuno suppressed populations. Because of the high burden of respiratory infections and pneumonia-related hospitalizations in SLE, 2 vaccines deserve special mention: the inactivated influenza vaccine and the pneumococcal vaccine.
Inactivated influenza vaccination. Prospective observational and randomized controlled trials of inactivated influenza vaccines have been conducted in patients with SLE. In the 1970s, several studies, including a randomized blind trial,10 examined the use of influenza vaccines in patients with SLE. These studies, and several subsequent trials, have found that the vaccine is generally safe.
Although influenza vaccination appears to be safe in SLE, a few patients may not mount a protective antibody response. A prospective nonrandomized study that involved 24 patients with SLE found that 75% of participants had a 4-fold or greater rise in antibody titer level to at least 1 of 3 influenza vaccine strains; 25% did not respond to any of the vaccine strains.11 All of the nonresponders were receiving immunosuppressive therapy. Although the vaccine is expected to protect most patients, a subset of patients may experience vaccine failure; active influenza infection therefore should be considered in vaccinated patients with characteristic symptoms.
Pneumococcal vaccine. Studies have reported a high incidence of pneumococcal infection in patients with SLE.12,13 Susceptibility to this infection probably has multiple causes in SLE, including immunosuppressive therapy, functional asplenia, and immunodeficiencies common in the disease (eg,complement and mannose-binding lectin deficiency).14
Vaccinating all immuno suppressed patients who have SLE provides an important opportunity to decrease the burden of this pathogen. Studies that examined the safety of pneumococcal vaccination have demonstrated that, like the influenza vaccine, it is safe for those with SLE. For example, a study of 73 patients with SLE monitored reactions to the administration of 3 simultaneous vaccines (pneumococcal, tetanus, and Haemophilus influenzae type B).15 No serious disease flares were detected, and equal numbers of patients had an increase or decrease in disease activity.
However, a few patients may not respond to the vaccine. In 1 study, 20% of patients did not form protective antibodies; both greater disease activity and increased immunosuppressive therapy were associated with a lack of response.16 The optimal vaccination strategy for this group of nonresponders requires further investigation; physicians should remain attuned to this possibility.
Bone health: Screening for osteoporosis
Patients with SLE are at increased risk for osteoporosis and fragility fractures. In 1 study, nontraumatic fractures occurred in 6.6% of 364 patients17; in another, 9% of 242 participants experienced a nontraumatic fracture after receiving a diagnosis of SLE.18 In addition to traditional risk factors, such as female sex, age, and early menopause, SLE-related risk factors also appear to play an important role; they include disease activity and chronic inflammation, vitamin D deficiency resulting from sun avoidance and, especially, longterm corticosteroid use.
Although osteoporosis is prevalent, screening for this condition remains suboptimal in SLE as well as in several other chronic conditions. A recent systematic literature review that examined osteoporosis screening in corticosteroid users found that in the vast majority of studies, fewer than one-third of eligible patients had undergone bone mineral density (BMD) testing (range, 1% to 47%).19 Another review that examined the use of pharmacotherapy for eligible patients receiving corticosteroids yielded similar findings20; in most of the 11 studies analyzed, only a few patients received adequate pharmacotherapy (calcium, vitamin D, antiresorptive or anabolic agents).
BMD testing and pharmacotherapy. Given the potential morbidity associated with nontraumatic fractures, improved care coordination for the prevention and treatment of osteoporosis in SLE is important. Baseline BMD testing should be performed on all patients at risk for osteoporosis, including those older than 65 years, those older than 60 years with a risk factor, and all those who are starting long-term corticosteroid therapy. Calcium and vitamin D supplementation should be recommended for patients in all these groups. In addition, those who are found to have significant osteopenia, osteoporosis, or a history of fragility fracture should be considered candidates for additional therapy with antiresorptive or anabolic agents, particularly if they are using corticosteroids long term.
Cancer screening: Increased risk in SLE?
Growing evidence suggests that patients with SLE are at increased risk for malignancy. The somewhat low prevalence of SLE has made identifying specific tumors that occur with higher incidence a challenge; however, the results of 2 recent large studies suggested that there probably is an increase in lymphoma and lung cancer.7,21
The reasons for the overall higher prevalence of malignancies in SLE remain unknown. Hypotheses include problems with immune surveillance, toxicities of medications, and viral agents associated with cancer.
Whether malignancies that are routinely screened for in the adult medical population are increased in SLE remains unclear. For example, some studies suggest an increased risk of breast cancer,22 but others suggest a decreased risk.21 Similarly, discrepant results have been reported for cervical cancer. 21,23 However, the incidence of cervical dysplasia in SLE appears to be increased,24 particularly in patients with a history of cytotoxic therapy.25 The use of human papillomavirus vaccination in at-risk patients with SLE has not yet been studied but may be a future prevention strategy for cervical dysplastic lesions.
Cancer screening strategies. No available research can answer directly the question of what is the optimal cancer prevention strategy in SLE. However, 2 general approaches should be considered: (1) physicians should remain vigilant for cancer in patients with SLE, and unexplained symptoms or signs should be investigated with malignancies, particularly lymphoproliferative diseases, kept in mind, and (2) although the optimal frequency to look for screenable malignancies in SLE remains unknown, patients should-at a minimum-receive cancer screening at intervals recommended for the general population.
There probably is significant room for improvement in this area. A recent study in Canada demonstrated that patients with SLE are significantly less likely than the general population to undergo routine cancer screening.26
CONCLUSIONS
Increased awareness and therapeutic advances have improved life expectancy in patients with SLE, but morbidity and mortality remain high. Understanding, preventing, and managing comorbidities and complications through interdisciplinary care can help improve outcomes.
References:
References
1. Urowitz MB, Bookman AA, Koehler BE, et al. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60:221-225.
2. Manzi S, Meilahn EN, Rairie JE, et al. Agespecific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408-415.
3. Manzi S, Wasko MC. Inflammation-mediated rheumatic diseases and atherosclerosis. Ann Rheum Dis. 2000;59:321-325.
4. Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331-2337.
5. Westerweel PE, Luyten RK, Koomans HA, et al. Premature atherosclerotic cardiovascular disease in systemic lupus erythematosus. Arthritis Rheum. 2007;56:1384-1396.
6. Scalzi LV, Ballou SP, Park JY, et al. Cardiovascular disease risk awareness in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:1458-1464.
7. Bernatsky S, Boivin JF, Joseph L, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550-2557.
8. Pradeep J, Watts R, Clunie G. Audit on the uptake of influenza and pneumococcal vaccination in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:837-838.
9. Bridges MJ, Coady D, Kelly CA, et al. Factors influencing uptake of influenza vaccination in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:685.
10. Williams GW, Steinberg AD, Reinertsen JL, et al. Influenza immunization in systemic lupus erythematosus: a double-blind trial. Ann Intern Med. 1978;88:729-734.
11. Abu-Shakra M, Press J, Varsano N, et al. Specific antibody response after influenza immunization in systemic lupus erythematosus. J Rheumatol. 2002;29:2555-2557.
12. Petros D, West S. Overwhelming pneumococcal bacteraemia in systemic lupus erythematosus. Ann Rheumatic Dis. 1989;48:333-335.
13. Naveau C, Houssiau FA. Pneumococcal sepsis in patients with systemic lupus erythematosus. Lupus. 2005;14:903-906.
14. Karim MY. Immunodeficiency in the lupus clinic. Lupus. 2006;15:127-131.
15. Battafarano DF, Battafarano NJ, Larsen L, et al. Antigen-specific antibody responses in lupus patients following immunization. Arthritis Rheum. 1998;41:1828-1834.
16. Elkayam O, Paran D, Caspi D, et al. Immunogenicity and safety of pneumococcal vaccination in patients with rheumatoid arthritis or systemic lupus erythematosus. Clin Infect Dis. 2002;34:147-153.
17. Petri M. Musculoskeletal complications of systemic lupus erythematosus in the Hopkins Lupus Cohort: an update. Arthritis Care Res. 1995;8:137-145.
18. Ramsey-Goldman R, Dunn JE, Huang CF, et al. Frequency of fractures in women with systemic lupus erythematosus: comparison with United States population data. Arthritis Rheum. 1999;42:882-890.
19. Morris CA, Cabral D, Cheng H, et al. Patterns of bone mineral density testing: current guidelines, testing rates, and interventions. J Gen Intern Med. 2004;19:783-790.
20. Solomon DH, Morris C, Cheng H, et al. Medication use patterns for osteoporosis: an assessment of guidelines, treatment rates, and quality improvement interventions. Mayo Clin Proc. 2005;80:194-202.
21. Parikh-Patel A, White RH, Allen M, Cress R. Cancer risk in a cohort of patients with systemic lupus erythematosus (SLE) in California. Cancer Causes Control. 2008 Apr 2. [Epub ahead of print].
22. Ramsey-Goldman R, Mattai SA, Schilling E, et al. Increased risk of malignancy in patients with systemic lupus erythematosus. J Investig Med. 1998;46:217-222.
23. Cibere J, Sibley J, Haga M. Systemic lupus erythematosus and the risk of malignancy. Lupus. 2001;10:394-400.
24. Tam LS, Chan AY, Chan PK, et al. Increased prevalence of squamous intraepithelial lesions in systemic lupus erythematosus: association with human papillomavirus infection. Arthritis Rheum. 2004;50:3619-3625.
25. Ognenovski VM, Marder W, Somers EC, et al. Increased incidence of cervical intraepithelial neoplasia in women with systemic lupus erythematosus treated with intravenous cyclophosphamide. J Rheumatol. 2005;31:1763-1767.
26. Bernatsky SR, Cooper GS, Mill C, et al. Cancer screening in patients with systemic lupus erythematosus. J Rheumatol. 2006;33:45-49.





