Article
Understanding Allopurinol-Induced DRESS Syndrome
Drug-induced rash with eosinophilia and systemic symptoms (DRESS) syndrome may be triggered by numerous chemically unrelated medications. Allopurinol-induced DRESS (A-DRESS) syndrome carries the highest mortality risk.
ABSTRACT: Drug-induced rash with eosinophilia and systemic symptoms (DRESS) syndrome may be triggered by numerous chemically unrelated medications. Allopurinol-induced DRESS (A-DRESS) syndrome carries the highest mortality risk. Two case reports reiterate the role of impaired renal function, accumulation of oxypurinol metabolite, and injudicious use of allopurinol in the development of A-DRESS syndrome. Viral triggers have been implicated; if they are a causative factor, the current treatment modality involving high-dose intravenous corticosteroids may not represent the best approach. The case reports serve as a reminder that allopurinol is not a benign drug and should be prescribed cautiously only for those with clear indications for its use. (J Musculoskel Med. 2011;28:171-184)
Drug-induced rash with eosinophilia and systemic symptoms (DRESS) syndrome may be triggered by numerous chemically unrelated medications that range from anticonvulsants to sulfonamides. Mild exanthema develops in 2% of patients who are receiving allopurinol, a drug that is used to treat patients with hyperuricemia and gout.1,2 Allopurinol hypersensitivity syndrome, a condition characterized by dermatitis, hepatitis, interstitial nephritis, and eosinophilia, was described in the literature and later renamed DRESS syndrome and then A-DRESS syndrome; it occurs in 0.4% of patients who are receiving allopurinol.1 The name is an umbrella term in that many other drugs have been known to induce a similar constellation of findings; A-DRESS syndrome is remarkable for the higher mortality rate with the use of allopurinol than with use of other agents (25% vs 10%).2
A-DRESS syndrome seems to be related to accumulation of the allopurinol metabolite oxypurinol.3 Impaired renal function and the use of thiazide diuretics, which favor accumulation of the metabolite, often coexist in patients in whom A-DRESS syndrome develops.2,4 However, because other chemically unrelated drugs whose metabolism is devoid of oxypurinol production also induce the syndrome, accumulation of oxypurinol alone is not sufficient to explain the pathogenesis of the condition; there must be another common inciting factor, probably immune-mediated, that triggers a yet unknown cascade of events.
A-DRESS syndrome is a rare entity that has not yet been addressed frequently in the medical literature. In this article, we offer 2 case reports and discussion to reiterate the role of impaired renal function, accumulation of oxypurinol, and injudicious use of allopurinol in the development of this syndrome.
Case 1
An 84-year-old African American woman presented with nausea, vomiting, abdominal pain, and diarrhea of 3 days' duration along with a disseminated pruritic rash. She had been hospitalized for an unrelated reason (urethral dilation) 6 weeks before the development of symptoms. It was during the previous hospitalization that the patient received a diagnosis of multifactorial anemia, stage 4 chronic renal failure, and gouty nephropathy, with an incidental solid 2.4-cm renal mass of unknown significance. She refused nephrectomy. The urethral dilation was uneventful, and the patient was discharged on a regimen of 100 mg/d of allopurinol. Her past medical history also included hypertension, coronary artery disease (CAD), sickle cell trait, chronic obstructive pulmonary disease, and gout.
On this admission, the patient complained of clear vomiting and diarrhea of 3 days' duration described as copious, brown, and continuing through the night. She described her abdominal pain as severe and colicky; it was located over her lower abdomen bilaterally. The patient denied any history of abdominal surgery, eating out, or sick contacts. According to the patient, the onset of pruritic rash coincided with the onset of the above symptoms.
The patient's medications included clopidogrel, amlodipine, metoprolol, acetaminophen, ferrous sulfate, vitamin D3 supplement, pantoprazole, hydralazine, and allopurinol (100 mg/d). She is known to be allergic to penicillin. Her family and social history were noncontributory. Results of a review of systems were negative.
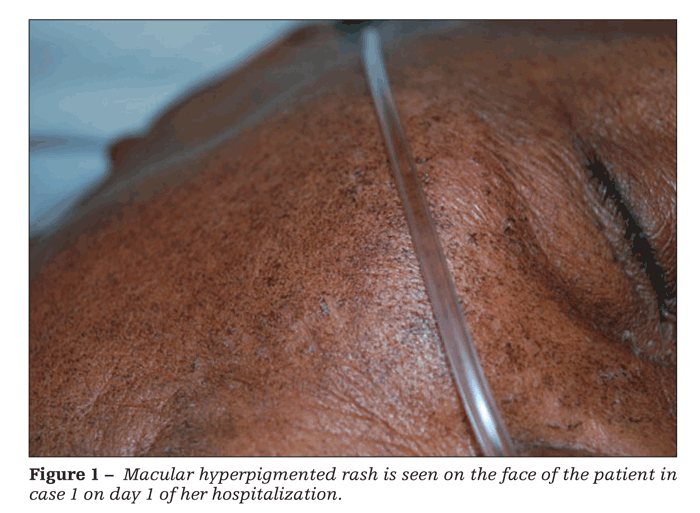
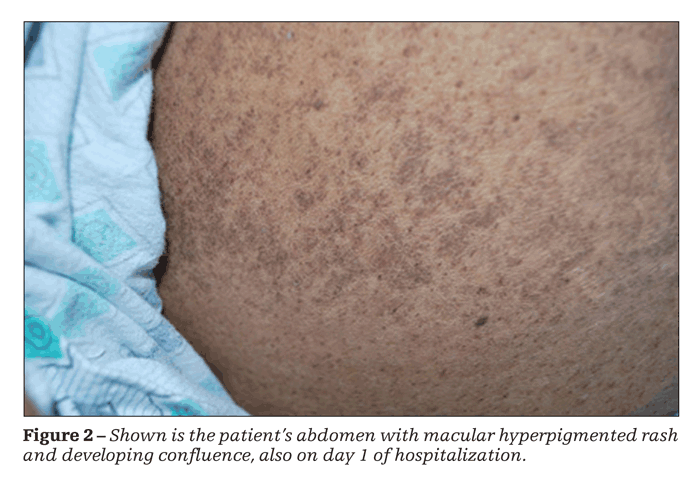
Physical examination revealed a febrile, ill-appearing patient; the remainder of her vital signs were unremarkable. A disseminated, fine, macular, hyperpigmented rash confluent in some areas was striking. The rash was present on her face (Figure 1), distal extremities, thorax, and abdomen (Figure 2). No adenopathy was noted.
Findings from the patient's heart, lung, and neurological examinations were unremarkable. Her abdomen was soft and diffusely tender; bowel sounds were diminished. No peritoneal signs or organomegaly was appreciated. Costovertebral angle tenderness was negative. Rectal examination results were within normal limits. Bilateral +1 pitting edema was present in both extremities.
Laboratory testing revealed creatinine and blood urea nitrogen (BUN) levels of 6.1 mg/dL and 103 mg/dL, respectively, and an anion gap of 20. Liver function test results showed the following values: aspartate aminotransferase (AST), 237 U/L; alanine aminotransferase (ALT), 205 U/L; alkaline phosphatase, 526 U/L; bilirubin, normal; total protein, 6.9 g/dL; and albumin, 2.9 g/dL. The patient's international normalized ratio (INR) was 1.05, and her partial thromboplastin time (PTT) was 25 seconds. Amylase and lipase levels both were 259 U/L, and the uric acid level was 8.4 mg/dL. Serum acetaminophen and salicylate levels were within normal range. Hepatitis panel results were negative.
The patient's complete blood cell count with differential (CBCD) was remarkable for leukocytosis (leukocyte count of 21,000/μL, with 59% eosinophils). Urinalysis showed cloudy urine with pyuria. Immunological test results were negative for antineutrophil cytoplasmic antibodies (cANCA and pANCA). Blood culture and sensitivity results were negative, as was Strongyloides IgG titer. However, the patient's urine was positive for Pseudomonas aeruginosa and her stool was positive for Clostridium difficile toxins A and B.
Findings on a chest x-ray film were within normal range. A CT scan of the patient's abdomen confirmed the presence of the previously found solid 2.4-cm renal mass (with no growth since the previous scan 6 weeks earlier).
Allopurinol and hydralazine were discontinued immediately, and the patient received several courses of intravenous corticosteroids, along with vancomycin for the C difficile infection. The rash, pruritus, and abdominal symptoms improved over the following few days.
On day 3 of hospitalization, however, the patient experienced the sudden onset of left-sided flank pain with overlying ecchymoses. Her hematocrit level dropped to 17%, and a repeated abdominal CT scan revealed spontaneous left-sided retroperitoneal hematoma. Blood transfusion was started, and the patient was transferred to the ICU. Because of the presence of multiple comorbidities and patient's age, she was treated for a myocardial infarction (MI) with medical management without percutaneous intervention. After that, her overall condition continued to improve.
On day 6 of hospitalization, the patient had no complaints and seemed comfortable in bed but her rash had changed from pruritic to vesicular. Clear, fluid-filled vesicles were present on her arms (Figure 3) and abdomen.
On day 8 of the patient's hospital stay, the rash became desquamative. Over the following several days, her symptoms and the laboratory findings returned to baseline and she was discharged. Allopurinol therapy was not restarted. We do not have a skin biopsy for this patient.
Case 2
An obese 61-year-old African American woman presented with generalized severely pruritic rash of 3 weeks' duration; left leg pain of 3 days' duration, aggravated by walking; and chronic leg swelling. Her past medical history was significant for stage 3 chronic kidney disease and severe peripheral arterial disease. She denied any history of falls, insect bite, recent travel, sick contacts, medication change, or use of new cosmetics. Results of a review of systems were negative.
The patient's past medical history also included hypertension, type 2 diabetes mellitus, diastolic congestive heart failure, and remote bilateral femoropopliteal bypass surgery. Her medications on admission included allopurinol, 200 mg/d; lisinopril; amlodipine; hydrochlorothiazide, 25 mg/d; metoprolol; aspirin; and clopidogrel. Allopurinol had been started for possible remote gout; the patient did not remember having recurrent attacks or having any of her joints aspirated. She had no known drug allergies. Her social and family histories were unremarkable.
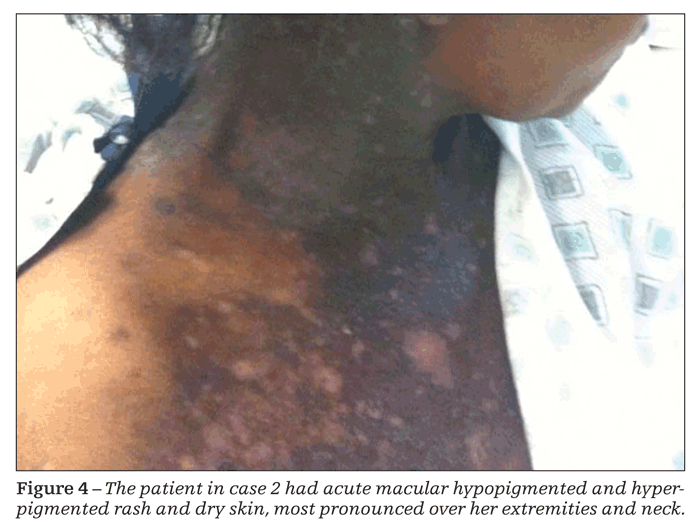
On physical examination, the patient seemed to be in no acute distress and had normal vital signs. Generalized macular rash-with areas of hyperpigmentation and hypopigmentation involving her palms and soles, along with dry skin, most pronounced over her extremities and neck-was striking (Figure 4). Findings from the heart, lung, and abdominal examinations were unremarkable.
The patient's left lower extremity was cooler than her right extremity. Her pulses were intact with Doppler studies. No neurological deficits were noted in either leg.
Laboratory testing revealed a CBCD remarkable for a white blood cell count of 12,000/μL, with 15.8% eosinophils; hemoglobin level, 9.5 g/dL; and hematocrit level, 29.3%. The patient's basic metabolic panel was remarkable for a creatinine level of 3.4 mg/dL (it was 1.9 mg/dL 4 months before this admission) and BUN level of 44 mg/dL. Her ALT, AST, bilirubin, and albumin levels and her INR and PTT were within normal range.
The combination of anemia, leg pain, and renal failure present in an older patient prompted a serum protein electrophoresis test to rule out multiple myeloma; the result was normal. Test results for rheumatoid factor and antinuclear antibodies were negative. Duplex ultrasonography of the patient's lower extremity was negative for deep venous thrombosis. Renal ultrasonography was negative for hydronephrosis. A chest x-ray film showed no acute cardiopulmonary processes. A skin biopsy revealed a lichenoid tissue reaction that was highly suggestive of drug hypersensitivity. Allopurinol was stopped, and treatment with intravenous corticosteroids was started.
On day 6 of admission, the patient experienced an acute drop in hemoglobin level, from 9.3 to 6.6 g/dL. This prompted the ordering of abdominal/pelvic/thigh CT scanning; the results were unremarkable. Esophagogastroduodenoscopy (EGD) showed erosive esophagitis, erosive gastritis, and mild hemorrhagic duodenitis. Pathological examination of an EGD biopsy specimen showed mild reactive gastropathy but no Helicobacter pylori. Disseminated intravascular coagulation panel results also were negative. The source of the patient's leg pain was investigated thoroughly (radiography, CT, MRI of the spine); no plausible cause was found.
Discussion

These 2 case reports share several important similarities but differ in other aspects. Both patients had recent exposure to allopurinol (for less than 8 weeks), exhibited diffuse pruritic rash, presented with acute deterioration of renal function, and showed significant eosinophilia. In addition, both patients were of African American background and incurred acute spontaneous drops in hemoglobin level. It is important to note, however, that the second patient had no clear indications for allopurinol use (tophaceous gout, asymptomatic hyperuricemia [serum uric acid level higher than 12 mg/dL], frequent acute gouty attacks, or gouty nephropathy).5,6 Diagnostic criteria for A-DRESS syndrome are shown in the Table.
One case report in the literature suggested a link between A-DRESS syndrome and acute coronary syndrome.7 The patient in our first case may have sustained an MI because of A-DRESS syndrome, or the MI may have represented an exacerbation of her underlying CAD resulting from acute anemia.
The acute spontaneous drop in hemoglobin level in both cases is clearly conspicuous. In the first case, this drop is easily explained by retroperitoneal hematoma. In the second case, however, no definite source of bleeding was found even with an extensive workup. The explanation could be pure red blood cell aplasia, which is associated with allopurinol use.8
Several case reports point toward viral causes as a trigger for DRESS syndrome and A-DRESS syndrome.9,10 The concept that a virus “sensitizes” or predisposes a person to a drug-induced reaction is not new. Classic examples would include the well-known amoxicillin drug reaction in a patient with Epstein-Barr virus–induced infectious mononucleosis11 and the high incidence of trimethoprim/sulfamethoxazole-related drug reaction in patients with AIDS. Viral causes are further suggested by generalized lymphadenopathy in many cases (not in the cases reported here) and by a mononucleosis-like picture.
Suzuki and associates9 tried to establish this relationship, particularly in connection with human herpesvirus 6 infection. Whether DRESS syndrome reactivates a quiescent viral infection or reactivation of a viral infection triggers DRESS or A-DRESS syndrome requires further investigation. Although the authors' results were tentative, the idea merits further study, especially because corticosteroids form the basis of treatment for patients with DRESS or A-DRESS syndrome-should viral causes prove to be the culprit, corticosteroids may not be the best approach to treatment and may in fact contribute to increased mortality.4,9,12,13
An association between impaired renal function and the use of thiazide diuretics in A-DRESS syndrome also is reported in the literature. Renal failure and use of thiazide diuretics probably favor accumulation of oxypurinol, which somehow triggers the cascade of events that culminates in the syndrome.14
One study suggested that patients with DRESS syndrome may represent a population of “slow-acetylators,”13 a term ingrained in the lexicon of drug-induced lupus erythematosus. Again, this idea requires further investigation.
The allopurinol hypersensitivity syndrome criteria that Singer and Wallace15 proposed in 1986 are broad, but they are the only ones available. The relative scarcity of the condition hampers the development of more precise diagnostic criteria.
Clinicians who encounter DRESS or A-DRESS syndrome are advised to obtain a skin biopsy and order a wide panel of viral titers. Contrary to prevailing thought, allopurinol is not a benign drug and should be prescribed cautiously only for those with clear indications for its use.6,15
The high mortality rate associated with A-DRESS syndrome, a relative lack of understanding of the mechanism involved, and no consensus on approaches to treatment render the syndrome a drug-related complication that most physicians would be inclined to avoid. However, febuxostat, a relatively new agent on the market, is a promising surrogate of allopurinol. This agent is the first one marketed in the United States to manage the hyperuricemia of gout since allopurinol was approved in 1964. Only time will tell whether febuxostat will replace allopurinol altogether. What is known thus far is that the drug has a much safer adverse-effect profile with no need for dose adjustments in patients with mild to moderate renal failure. The cost of this new selective xanthine oxidase inhibitor remains high, hindering more widespread use at this point.16
References
1. Lang PG Jr. Severe hypersensitivity reactions to allopurinol. South Med J. 1979;72:1361-1368.
2. Gutirrez-Macas A, Lizarralde-Palacios E, Martnez-Odriozola P, Miguel-De la Villa F. Fatal allopurinol hypersensitivity syndrome after treatment of asymptomatic hyperuricaemia. BMJ. 2005;331:623-624.
3. Pacher P, Nivorozhkin A, Szab C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87-114.
4. Arellano F, Sacristn JA. Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother. 1993;27:337-343.
5. Becker MA. Treatment of gout. Uptodate Online Version 13.2. Retrieved August 20, 2005 from the World Wide Web: http://www.uptodate.com.
6. Lee HY, Ariyasinghe JT, Thirumoorthy T. Allopurinol hypersensitivity syndrome: a preventable severe cutaneous adverse reaction? Singapore Med J. 2008;49:384-387.
7. Chan YC, Tay YK, Ng SK. Allopurinol hypersensitivity syndrome and acute myocardial infraction-two case reports. Ann Acad Med Singapore. 2002;31:231-233.
8. Lin YW, Okazaki S, Hamahata K, et al. Acute pure red cell aplasia associated with allopurinol therapy. Am J Hematol. 1999;61:209-211.
9. Suzuki Y, Inagi R, Aono T, et al. Human herpesvirus 6 infection as a risk factor for the development of severe drug-induced hypersensitivity syndrome. Arch Dermatol. 1998;134:1108-1112.
10. Ichiche M, Kiesch N, De Bels D. DRESS syndrome associated with HHV-6 reactivation. Eur J Intern Med. 2003;14:498-500.
11. Mardivirin L, Valeyrie-Allanore L, Branlant-Redon E, et al. Amoxicillin-induced flare in patients with DRESS (Drug Reaction with Eosinophilia and Systemic Symptoms): report of seven cases and demonstration of a direct effect of amoxicillin on Human Herpesvirus 6 replication in vitro. Eur J Dermatol. 2010;20:68-73.
12. Chopra S, Levell NJ, Cowley G, Gilkes JJ. Systemic corticosteroids in the phenytoin hypersensitivity syndrome. Br J Dermatol. 1996;134:1109-1112.
13. Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206:353-356.
14. Elion GB, Benezra FM, Beardmore TD, Kelley WN. Studies with allopurinol in patients with impaired renal function. Adv Exp Med Biol. 1980;122A:263-267.
15. Singer JZ, Wallace SL. The allopurinol hypersensitivity syndrome: unnecessary morbidity and mortality. Arthritis Rheum. 1986;29:82-87.
16. Ernst ME, Fravel MA. Febuxostat: a selective xanthine-oxidase/xanthine-dehydrogenase inhibitor for the management of hyperuricemia in adults with gout. Clin Ther. 2009;31:2503-2518.

Real-World Study Confirms Similar Efficacy of Guselkumab and IL-17i for PsA



