Publication
Article
Cardiology Review® Online
ADHD Medications and Risk of Serious Cardiovascular Events

Karla K. Quevedo, MD
Review

Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306:2673-2683.
Between 2001 and 2010 there was an increased use of medications for treatment of attention deficit/ hyperactivity disorder (ADHD) in adults.1 Drugs prescribed for ADHD
are in the stimulant classes methylphenidate and amphetamine, and also include a nonstimulant agent, atomoxetine, a selective norepinephrine reuptake inhibitor. The purpose of this study was to examine whether current use of medications for ADHD is associated with increased risk of myocardial infarction (MI), sudden cardiac death (SCD), or stroke in adults aged 25 through 64 years.
Study Details
Data were obtained from 4 study sites: Vanderbilt University (Tennessee State Medicaid data), Kaiser Permanente (KP) California (northern and southern KP region), Optum Insight Epidemiology (data from a large health insurance plan), and the HMO Research Network (Harvard Pilgrim Health Care; Fallon Community Health Plan; Group Health Cooperative of Puget Sound; HealthPartners; KP Georgia; KP Northwest; and KP Colorado). Data sites had geographic and sociodemographic diversity, and similar computerized data structures. The start date for computerized data ranged from 1986 to 2002. Follow-up concluded at the end of 2005 and mortality searches were conducted using complete state death records and the National Death Index. Participants were aged 25 through 64 years with at least 12 months of continuous health plan coverage and pharmacy benefits before cohort entry (time zero). Exclusion criteria were 1 or more of the following: sickle cell disease, cancer (other than nonmelanoma skin cancer), HIV infection, organ transplant, liver failure/hepatic coma, endstage renal disease, respiratory failure, or congestive heart failure. Medication use was based on prescription fills from electronic pharmacy records. ADHD medications included stimulant-class medications (methylphenidate, amphetamines,
pemoline) and atomoxetine. Current use was defined as the period between prescription start date and end of days supply. Indeterminate use was the first 89 days after end of current use. Former use began at 90 days after end of current use and ended at 364 days after last current use. Remote use was more than 364 days since end of last days supply.
In order to evaluate for study end points of SCD, stroke, and MI, medical records, including hospitalizations, reports from emergency medical services, autopsies, and death certificates, were requested for all potential SCDs and strokes for assessment by trained adjudicators. MI was defined as an acute event involving hospitalization with changes in cardiac enzyme levels and either symptoms or electrocardiographic changes. SCD was defined as witnessed sudden death in a community setting, preceded by typical symptoms of cardiac ischemia. Stroke was defined as an acute neurologic deficit that persisted more than 24 hours. In the case of MI, of the 371 cases, 353 (95%) were confirmed by adjudication.
For potential SCD cases without available or adequate hospital or autopsy records, an ICD-9/ICD-10 code— based definition was used, and those cases were included in the study. For SCD cases, 157 were based on code definition, and 139 were confirmed by clinical adjudication. Probable strokes based on ICD-9/ICD-10 code definition
were also included in the study.
For stroke cases, 451 were confirmed by adjudication and 124 were based on code definition. Secondary analyses included all electronically identified SCD or strokes except those confirmed as nonevents by adjudication. To avoid confounder variables in cardiovascular disease (CVD) risk, a cardiovascular risk score (CRS) was constructed. The CRS was based on inpatient and outpatient diagnosis and pharmacy records and included CVD and medications, mental health conditions and use of psychotropic medications, other health conditions and medications, and health care utilization. For each end point, a separate score was created from a Poisson regression model among all patients, adjusted for ADHD medications and matching variables. In primary analyses, several CRS variables not thought to be on the
causal pathway between medication use and the outcomes were treated as time varying.
In secondary analyses, all CRS variables were based on diagnoses or medication use in the 364 days prior to cohort entry and fixed at baseline. For the new-user analyses, CRS was used for comparisons of current versus remote use, and a propensity score was constructed for current versus nonuse of ADHD medications at cohort entry.
Follow-up began at cohort entry and ended at 1 of the 4 end points: death, end of insurance coverage, day before 65th birthday, or end of study period. Poisson regression was used to estimate the association of ADHD medication use with risk of serious cardiovascular events, while adjusting for potentially confounding variables.
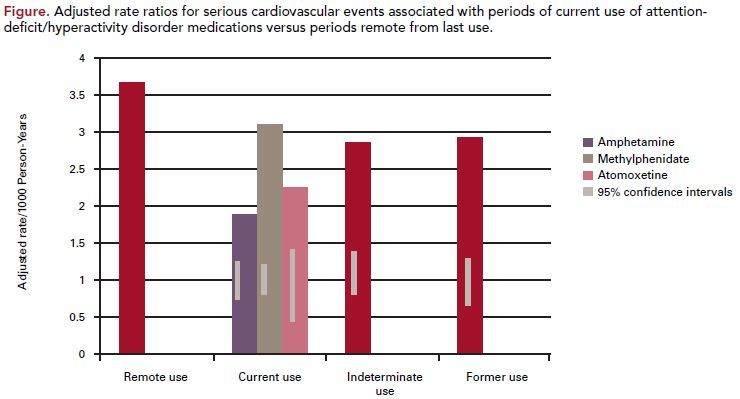
The study included a total of 443,198 adults; of these, 150,359 were users of ADHD medications at baseline. Methylphenidate accounted for 45% of current use; amphetamine for 44%; atomoxetine for 8%; and pemoline for 3%. During 806,182 person-years of follow-up, 1357 cases of MI, 296 cases of SCD, and 575 cases of stroke occurred. There were 107,322 personyears of current use, with a crude incidence per 1000 person-years of 1.34 for MI, 0.30 for SCD, and 0.56 for stroke. In analysis adjusted for matching variables only, the relative risk (RR) of MI, SCD, or stroke for current versus nonuse of ADHD medication was 0.97. After adjusting for the CRS, the RR was slightly lower at 0.83. Results were similar for specific medications and across end points. Among ever users of ADHD medications, the adjusted RR of serious cardiovascular events was nearly the same during periods of current use as during follow-up periods more than 1 year after use ended. For the new-user
analyses, RRs for current versus remote use were close to 1.0 for end point variables.
As noticed in the study, there was no evidence of an increased risk of MI, SCD, or stroke associated with ADHD medication use, compared with nonuse
or remote use. Rate ratios did not appear to be influenced by prior CVD or by prior non-ADHD psychiatric conditions (Figure).
The study had several limitations. The use of ADHD medications was based on electronic records of prescriptions dispensed, which does not mean medications that were actually consumed, and so may not represent actual periods of use. The study didnot include adults older than 65 years, so results cannot be generalized to this population. Despite the large size of the study, it had only moderate power for several comparisons, including current versus remote use in the newuser analysis and in comparisons for individual drugs. Medical records were not available to confirm all SCD and stroke cases, and ICD-9/ICD-10 code-based definitions were used for suspected cases, so misclassification could have occurred. There appears to be a modest amount of healthyuser bias influencing RR comparisons
of current use versus nonuse. In sensitivity analyses, the potential source of this bias was attributed to a higher percentage of users that were white and college educated.
The strengths of the study include its large size, use of cardiovascular risk score to control for CVD risk, and use of sensitivity analyses to control confounding
variables. In this cohort study of young and middle-aged adults, the current or new use of ADHD medication, compared with nonuse or remote
use, was not associated with an increased risk of serious cardiovascular events. A significant risk cannot be excluded, given limited power and lack of
complete information.
References
1. Medco Health Solutions, Inc. America’s State of Mind. November 16, 2011. http://medco.mediaroom.com.
2. Polanczyk G, de Lima MS. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry.2007;164:942-948.
3. Fayyad J, De Graaf R, Kessler R, et al.Cross national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190:402-409.
4. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163:1149-1152. 5. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit hyperactivity disorder beget later substance abuse? a meta-analytic review of the literature.
Pediatrics. 2003;111:179-185.
6. Klein-Schwartz W. Abuse and toxicity of methylphenidate. Curr Opin Pediatr.2002;14:219-223.
7. Volkow ND, Wang GJ, Fowler J, et al. Cardiovascular effects of methylphenidate in humans are associated with increases of dopamine in brain and of epinephrine in plasma. Psychopharmacology. 2003;166:264- 270.
8. Samuels JA, Franco K, Wan F, et al. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double, randomized, cross over trial. Pediatr Nephrol. 2006;21:92-95.
9. Findling RL, Biederman J, Wilens TE, et al. Short- and long-term cardiovascular effects of mixed amphetamine salts extended release in children. J Pediatr. 2005;57:147-154.
10. Spencer T, Biederman J, Wiles, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity
disorder. Biol Psychiatry. 2005;57:56-63.
11. Donner R, Michaels MA, Ambrosini PJ. Cardiovascular effects of mixed amphetamines salts extended release in the treatment of school-aged children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:706-712.
12. Nissen SE. ADHD drugs and cardiovascular risk. N Engl J Med. 2006;354:1445-1448.
13. Wiles TE, Prince JB, Spencer TJ, et al. Stimulants and sudden death: what is a physicianto do? Pediatrics. 2006;118:1215-1219.
14. Villalba L, Racoosin J. Postmarketing safety review of sudden death during treatment with drugs used to treat ADHD. 2006. Food and Drug Administration. http://www.
fda.gov/ohrms/dockets/ac/06/briefing/2006-4210b_07_01_safetyreview.pdf. Published 2006.
15. Wernicke JF. Cardiovascular effects of atomoxetine in children, adolescents and adults. Drug Safety. 2003;26:729-740. 16. Berenson GS, Srinivsan SR, Bao W, Newman WP 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults: the Bogalusa Heart Study. N Engl J Med. 1998;338:1650-1656.
17. Lewington S, Clarke R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
18. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity
disorder: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young, Congenital Cardiac Defects Committee and the
Council on Cardiovascular Nursing. Circulation. 2008;117:2407-2423.
COMMENTARY
ADHD Medications and Risk of Adverse Cardiovascular Events
ADHD is one of the most common mental disorders, with a worldwide mean prevalence of around 5% in children2 and 3% among adults.3 The expanded use of ADHD medications in adolescents and adults and the use of new stimulants and nonstimulant compounds have contributed to bringing new attention to short- and long-term risks for pharmacologic treatment of ADHD.4,5 Stimulants are sympathomimetic agents that increase noradrenergic and dopaminergic transmission. An effect on heart rate and blood pressure can be considered a feature of their pharmacologic activity.6,7 The magnitude of the increase over placebo is approximately 2 to 6 beats per minute (bpm) for heart rate, 2 to 4 mm Hg for systolic blood pressure, and 1 to 3 mm Hg for diastolic pressure.8 Some studies, however, in spite of large sample sizes, did not find differences in blood pressure or heart rate compared with placebo.9,10 Open-label studies of children during long-term treatment indicate that thesechanges in heart rate and blood pressure tend to persist, a sign that full tolerance does not develop during chronic treatment.9,11 Another important association of stimulants
is with sudden cardiovascular death.12,13 The cases of SCD are reported to the US Food and Drug Administration’s Adverse Event Reporting System (AERS). From
January 1992 to February 2005, 20 cases of sudden death during treatment with amphetamine were reported (14 children, 6 adults). During the same period, 18 cases of sudden death during treatment with methylphenidate were reported (14 in children, 4 in adults).14 The estimated rates of sudden death based on these reports is below the background rates in the general population, but only a percentage of adverse events are reported to the AERS, so an accurate incidence rate cannot be determined.
A review of 5 placebo-controlled clinical trials involving children, adolescents, and adults treated with therapeutic doses of atomoxetine up to 10 weeks confirmed an increase of 5 to 9 bpm in mean heart rate.15 Systolic blood pressure was increased in adults. These changes occurred in the first weeks of treatment and stabilized afterward, with no further increases during long-term treatment of 1 year and longer.15 Between November 2002 and February 2005, 7 cases of SCD during atomoxetine treatment were reported to the AERS (3 children, 4 adults), but no evidence of causality can be derived from these cases, as there were multiple confounders.
Stimulants and atomoxetine have proved to have pressor and chronotropic cardiac effects, which usually are not clinically significant in the short term, but their possible significance for the long term is still under investigation. Despite the lack of a public health approach to hypertension in younger people, it is recognized that intimal plaque formation may occur as a result of hypertension in children,16 and in the long term even small increases of blood pressure are associated with an increased incidence of cerebrovascular disease in adults.16 Other consequences include end organ damage such as left ventricular hypertrophy, retinopathy, and nephropathy with proteinuria. In adults, a 2—mm Hg lowering of systolic blood pressure would result in 10% lower stroke mortality and 7% lower mortality from ischemic heart
disease.17
Although a causal link between therapeutic stimulant use and SCD has not been established, there are concerns that treatment may increase the risk for SCD in patients who have structural cardiac abnormalities, so that pretreatment assessment and clinical screening currently is recommended in the pediatric population. The American
Heart Association’s scientific statement recommends that in children and adolescents receiving ADHD medications, a careful history, physical exam, electrocardiogram,
and pediatric cardiologist consult must be obtained before stimulant medications are started. It is also recommended that regular cardiovascular monitoring be conducted at each visit via physical exam and questioning about potential cardiac symptoms and new family history.18
Even though a causal event effect has not been proved in multiple studies, it is possible that because of their sympathomimetic activity increasing heart rate and blood pressure, stimulants and atomoxetine may increase the risk for cardiovascular disease at usual therapeutic doses. Considering the increasing use of stimulants for the treatment of ADHD in adults, it is important to investigate further cardiovascular effects, with special attention to patients who have risk factors for heart disease, such as hypertension, atherosclerosis, smoking, or concomitant use of other drugs. Because it will be very difficult to assess the true cardiac risks of stimulants, consensus recommendations are needed to determine whether screening is required before starting ADHD treatment, what constitutes an appropriate screening, and which adults can be treated cautiously with stimulant medication. The AHA statement provides the first scientific statement for practitioners who treat children and adolescents with
ADHD; however, no statement is available for continuing monitoring in adults with ADHD. It seems that the next step would be the establishment of guidelines with proper recommendations for adults who are going to be started or are continuing to take ADHD medications, with special emphasis for those adults who already have
cardiovascular risk factors.
About the Author
Karla K. Quevedo, MD, is a third-year resident in internal medicine at Texas Tech University Health Sciences Center in El Paso, Texas. Dr Quevedo received her MD at the Universidad San Martin de Porres in Peru in 2003. She was chief resident in internal medicine at Guillermo Almenara Hospital in Lima, Peru. Her areas of interest are general cardiology, preventive cardiology, and heart failure and biomarkers.
