Article
After COVID-19, Psoriasis Patients Prefer Biologics with Infrequent Administration, Fewer Medical Visits
Author(s):
This research from Japan on the shifting preferences for individuals with psoriasis in the pre and post-pandemic era may indicate the effects of such events on treatment options.
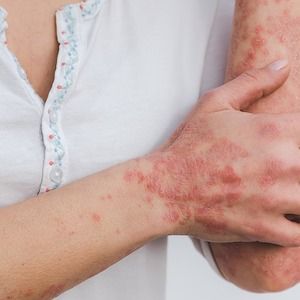
Individuals with psoriasis prefer biologic treatment with a more infrequent administration route and medical visit schedule, recent findings indicate, contrasting with prior data which indicated that the highest importance for patients in treatment preference was simply efficacy.1
As clinicians choose biologics for their patients, many different factors such as patient background and biologic characteristics are considered. As such, there are not any globally established biologic selection criteria.2
The findings resulted from an assessment of the preferred attributes for selection of biologics by psoriasis patients and the research was authored by Asako Itakura, from the Department of Dermatology at Teikyo University School of Medicine in Tokyo.
“In October 2021, when the survey was conducted, coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 was prevalent in Japan and globally, and a declaration of a state of emergency by the Japanese government was in effect from April until the end of September 2021,” Itakura and colleagues wrote. “Therefore, this study reflects the impact of the COVID-19 outbreak on patient preferences for biologics in psoriasis treatment.”
Background and Findings
The investigators sought to determine patient preferences for biologic use in psoriasis treatment and to analyze the influence of social and clinical backgrounds on these different preferences. A web-based questionnaire survey using a Discrete Choice Experiment (DCE) was provided by the research team from October 4, 2021, to October 8, 2021.
The participants were patients with psoriasis registered in the patient panel owned by INTAGE Healthcare Inc in Tokyo, Japan. The panel included around 4,000 individuals who had received psoriasis treatment within the previous year, with 2,581 reported to be undergoing treatment currently as of August 2021.
The investigators conducted their survey among adult patients (age 20 years or above) who had been diagnosed with psoriasis and were also being treated for the condition. Patients who did not agree to participate were excluded from the trial.
In order to ensure an adequate sample size, the research team aimed to collect 400 or more responses, considering previous studies and the requirement for at least 300 effective respondents.
The team utilized a questionnaire survey titled the Discrete Choice Experiment (DCE) in order to evaluate patient preferences regarding biologic treatments for the skin condition. Study participants were presented with 2 hypothetical choice scenarios, each combining different attributes of biologics.
These attributes were determined based on prior studies on psoriasis patients and which had examined patient preferences for biologic treatments.
The attributes included efficacy at 1 year, efficacy on skin symptoms and other manifestations, indications for different patient types, risk of serious infections requiring hospitalization, incidence of injection site reactions, administration route and visits, and co-payment.
The levels of each of the attributes were decided based upon the characteristics of available biologics on the market. Participants were asked to select the most favorable choice scenario from the two presented.
The study employed a discrete choice experiment (DCE) methodology to assess patient preferences for various attributes and levels.
Participants were assigned 18 DCE questions, each with the aforementioned 2 hypothetical scenarios. These scenarios were developed using an orthogonal array and then presented with visual representations and textual descriptions to enhance participant understanding and engagement.
The investigators also gathered socioeconomic and clinical data from the study’s respondents. Before the main study, a pilot survey involving 33 study participants was done to validate the sample size and assess respondents' comprehension of their questionnaire.
Overall, the research team assessed data from 357 psoriasis patients, and their results demonstrated some of the key factors influencing the preference for biologic medications.
The most important attributes for the patients when selecting biologics were found to be method of administration and the frequency of visits, followed then by the risk of severe infections that might necessitate hospitalization.
The team did add that some variations were seen within specific subgroups. Still, the results were notable, given their divergence from prior studies where the primary emphasis was on the effectiveness of the drug.
“These results may reflect the personal and social impact of the coronavirus disease outbreak at the time of the survey,” they wrote. “The results of this study might help physicians properly select biologics that satisfy psoriasis patients' needs, leading to better treatment adherence.”
References
- Tada, Y, Itakura, A, Hosono, K, Kawamura, T. Psoriasis patient preferences for the use of biologics during the coronavirus era. J Dermatol. 2023; 50: 596– 607. https://doi.org/10.1111/1346-8138.16703.
- Saeki H, Terui T, Morita A, Sano S, Imafuku S, Asahina A, et al. Japanese guidance for use of biologics for psoriasis (the 2019 ver-sion). J Dermatolog Treat. 2020;47:201–2.





