Publication
Article
Cardiology Review® Online
Antihypertensive Drugs and Risk of Cancer
Author(s):


Alison L. Bailey, MD
REVIEW
Bangalore S, Kumar S, Sverre Kjeldsen E, et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 2011;12:65-82.
H
ypertension is a commonly encountered clinical problem and affects about 1 in 3 adults in the United States. While lifestyle changes are advocated as the initial treatment of hypertension, most patients will require antihypertensive drugs to achieve recommended blood pressure goals.1 Over the years, various classes of antihypertensive medications have been linked to an increased risk of cancer.2-6 The current study analyzed the existing data regarding antihypertensive drugs and risk of cancer and cancer-related mortality.7
Study Design
This meta-analysis included 70 randomized, controlled trials of antihypertensive drugs spanning the years from 1950 to 2010. Only trials that enrolled more than 100 patients, had 1 year or more of follow-up, and were able to report on the end points of interest (cancer and cancer-related mortality) were included. A total of 324,168 participants were enrolled in these trials, with a mean follow- up of 3.5 years. Drugs were categorized into eight comparison categories: placebo, angiotension receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACEi), β-blockers, calcium channel blockers (CCBs), diuretics, ACEi plus ARBs, and other controls (nonplacebo active treatment). The ACEi plus ARB combination was evaluated as a discrete drug category because of the recent study suggesting increased cancer risk with this combination as well as ARBs alone.6 Direct comparisons were available between many of the drug classes.
The traditional meta-analyses directly compared cancer risk and cancer-related mortality within individual drug classes. Additionally, the authors chose to analyze the trial data using multiple comparison (network) meta-analyses due to the lack of direct comparisons in many of the antihypertensive trials. This statistical tool allows indirect comparisons between drugs that are not directly compared in the individual trials. Finally, trial sequential analysis was performed for the direct meta-analyses to test for consistency of effect between the direct and multiple comparison analyses.
For the multiple comparison metaanalyses, the risk of cancer with any single class of antihypertensive therapy was not significantly different than with placebo (2.02%; 95% confidence interval [CI], 1.67-2.35) with either of the statistical models used (fixed effect and random effects). The risk of cancer for each individual classes of drug was: ARBs (2.04%; odds ratio [OR], 1.01; 95% CI, 0.93-1.09); ACEi (2.03%; OR, 1.00; 95% CI, 0.92-1.09); β-blockers (1.97%; OR, 0.97; 95% CI, 0.88-1.07); CCBs (2.11%; OR, 1.05; 95% CI, 0.96-1.13); diuretics (2.02%; OR, 1.00; 95% CI, 0.90-1.11); or other controls (1.95%; OR, 0.97; 95% CI, 0.74-1.24) versus placebo. There was a small increased risk of cancer seen with the combination of an ACEi plus ARB in the fixed effect model (2.30%; OR, 1.14; 95% CI, 1.02- 1.28) that was not seen in the random effects model. There was no increased risk of cancer-related mortality compared with placebo (1.32%; 95% CI, 1.01- 1.63) with any of the antihypertensive classes evaluated in this analysis (Table).
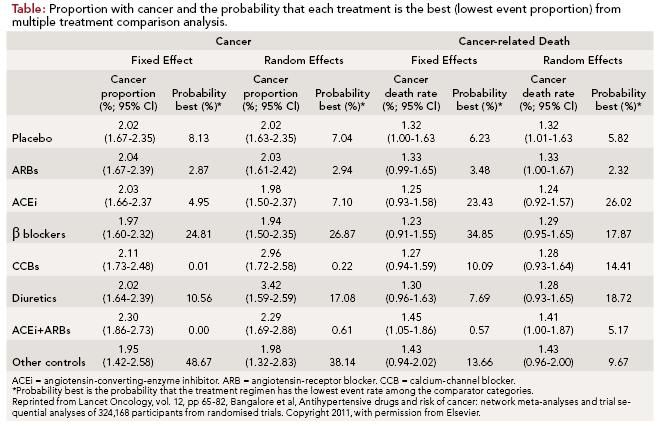
For the direct meta-analyses, antihypertensive drug classes were directly compared against all possible comparators. This analysis included data from 21 randomized trials and more than 120,000 patients. For most antihypertensive drug classes, there was no increased risk of either cancer or cancer-related mortality (ARBs, ACEi, β-blockers, and diuretics). However, an increased risk of cancer was seen with CCBs (OR, 1.06; 95% CI, 1.01-1.12; P = 0.02) as well as the combination of ACEi plus ARB (OR, 1.14; 95% CI, 1.04-1.24; P = 0.004) without an increased risk of cancer-related death.
To further support the findings seen in the meta-analyses, a trial sequential analysis was performed. The trial sequential analysis suggested no evidence of a substantially increased relative risk (up to 10%) in either cancer or cancerrelated mortality with any individual class of antihypertensive. However, there was firm evidence to suggest at least a 10% increase in the relative risk of cancer with the combination of an ACEi plus ARB. There were not enough data to analyze for cancer-related mortality with this combination of drugs.
Subgroup analyses of trials with longer follow-up (≥3 years), with low risk of bias, and that used cancer as a prespecified outcome did not differ significantly from the main results for either cancer risk or cancer-related mortality. Additionally, specific subclasses of antihypertensive drugs were evaluated without any significant difference found in cancer risk or cancer-related mortality (low versus high tissue-affinity ACEi, cardioselective versus non-cardioselective β -blockers, dihydropyridine versus non-dihydropyridine CCBs, telmisartan versus non-telmisartan ARBs).
In summation, the analyses do not suggest even a small relative increase in the risk of cancer or cancer-related death with the use of common antihypertensives (ARBs, ACEi, β-blockers, diuretics, or CCBs). However, an increased risk of cancer with the ACEi plus ARB combination cannot be excluded.
References
1. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. [published online ahead of print May 14, 2003] JAMA. 2003;289(19):2560-2572.
2. The Boston collaborative drug surveillance program. Reserpine and breast cancer. Lancet. 1974;304:669-671.
3. Kaplan NM. Do calcium antagonists cause death, gastrointestinal bleeding, and cancer? Am J Cardiol. 1996;78:932-933.
4. Finkle WD, McLaughlin JK, Rasgon SA, Yeoh HH, Low JE. Increased risk of renal cell cancer among women using diuretics in the United States. Cancer Causes Control. 1993;4:555-558.
5. Kanamasa K, Kimura A, Miyataka M, Takenaka T, Ishikawa K. Incidence of cancer in postmyocardial infarction patients treated with short-acting nifedipine and diltiazem. Secondary Prevention Group. Cancer. 1999;85:1369-1374.
6. Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11:627-636.
7. Bangalore S, Kumar S, Sverre Kjeldsen E, et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 2011;12:65-82.
8. Grossman E, Messerli FH, Goldbourt U. Antihypertensive therapy and the risk of malignancies. Eur Heart J 2001;22:1343-1352.
9. Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003;362:759-766.
10. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547-1559.
COMMENTARY
Reconsidering Antihypertensives’ Risks
R
eports of increased cancer risk with antihypertensive therapy are not new, but continue to raise clinical concern.6,8 This is of interest from a public health perspective, as both hypertension and cancer are extremely common in the United States. This large meta-analyses of commonly used antihypertensive classes revealed no increased risk of cancer or cancerrelated mortality with any individual drug class. However, an increased risk of cancer was seen with the combination of an ACEi plus ARB in several of the analyses undertaken (OR, 1.14; 95% CI, 1.02-1.28).7
The results of this study are in contrast to the most recent publication linking an antihypertensive class to increased risk of cancer as well as older trial data that associated ARBs with increased risk of cancer.6,9,10 Sipahi et al performed a meta-analysis of ARBs and found an increased risk of cancer with the use of ARBs (meta- analytic RR, 1.08; 95% CI, 1.01-1.15; P = 0.016) without any increase in cancer related mortality.6 In the current study, however, there was no increased risk of cancer with ARBs (OR, 1.01; 95% CI, 0.93- 1.09) and the results were similar across the various statistical methods used.
These two trials used different statistical methods. Sipahi et al used direct comparison metaanalyses, while the current study added multiple comparison analyses and trial sequential analysis to the more traditional direct meta-analyses. Additionally, the current study has a larger number of patients and trials represented in the direct analyses than the Sipahi study (21 trials, >120,000 patients versus 5 trials, 61,590 patients).
Several limitations to this study deserve mention. The majority of the trials included (>75%) did not evaluate cancer as a prespecified outcome. The proportion of patients with cancer was 2.02%. However, the proportion of patients with cancer was higher (4.72%) in those trials where cancer was a prespecified end point. Thus, cancer risk is most likely underreported in the majority of these trials and the results might be different if focused reporting had been present. The trials included in this analysis represent a diverse and heterogeneous mix of people and drug usage.
A more robust evaluation would include patient-level data so that additional factors could be analyzed (dose of drug, compliance rate, tobacco use). Additionally, most carcinogens require prolonged exposure before a direct cancerous effect is observed; thus the short follow- up of this trial (mean, 3.5 years) likely precludes true assessment of cancer risk related to drug use. Finally, although this was a large study, it was still underpowered to detect very small differences in cancer risk (<5%). An increased risk of this magnitude would be difficult to recognize in a trial, but may be clinically significant due to the high prevalence of hypertension and antihypertensive therapy.
While the analyses in this trial are not perfect, the findings suggest no increased risk of cancer with any single antihypertensive class. However, ACEi plus ARB usage was associated with an increased risk of cancer. Although these data are mostly hypothesis generating, perhaps we should reconsider the use of ACEi in combination with ARB for routine therapy of hypertension.
About the Author
Alison L. Bailey, MD, is Assistant Professor of Medicine and Director of Cardiac Rehabilitation at the Gill Heart Institute, Division of Cardiovascular Medicine, at the University of Kentucky in Lexington. She is also Associate Director of the Cardiovascular Fellowship Program. Dr Bailey received her MD from the University of Kentucky College of Medicine and completed her residency and fellowship at University of Kentucky Chandler Hospital. Her clinical interests include cardiovascular disease in women and cardiovascular disease prevention. Dr Bailey has been published in numerous peer-reviewed medical journals.
