Article
Biologics Efficacy, Safety Not Significantly Impacted by Comorbidities in Older Adults with Psoriasis
Author(s):
New data indicates that biologic treatments for psoriasis patients were found not to be significantly affected by comorbidities in older patients, widening the understanding of biologic use in different subgroups.
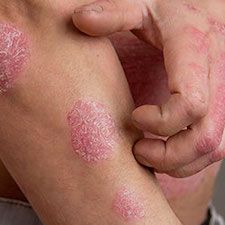
Neither the efficacy nor the safety of biologics are heavily affected by comorbidities when used in treatment of elderly psoriasis patients, recent findings suggest.1
Psoriasis is a chronic inflammatory disease that commonly affects older patients, with a prevalence of 0.14% to 1.99% in those over 65 years of age.2 Systemic therapy is indicated for moderate-to-severe psoriasis, but treating older patients may be challenging as a result of both frailty and comorbidities.
That being said, there is a lack of data on the use of biological therapies for psoriasis in older and frail patients, as they are often excluded from clinical studies.3 Therefore, available data on the use of biological agents in elderly patients mainly results from real-life studies, case reports, and case series.
This new study, in which elderly psoriatic patients were assessed, was authored by François Rosset, from the Department of Medical Sciences Dermatologic Clinic at the University of Turin in Italy.
“Herein, we present a case series of elderly psoriatic patients treated with biological therapy, evaluating the impact of different comorbidities in a real-life clinical setting of a tertiary level university hospital,” Rosset and colleagues wrote.
Background and Findings
From 2019 - 2022, there were 1,600 psoriasis patients treated with anti-interleukin (IL)-23 or anti-IL-17 agents. Out of the total number of patients, 280 were found to have been aged 65 and up.
The patients were divided by the investigators into 2 groups based on the presence of comorbidities at the time of diagnosis. The first group was made up of 219 patients who reported at least one comorbidity, while the other group was made up of 61 patients who denied having any comorbidities.
The comorbidities included chronic diseases such as hematological, cardiological, gastroenterological, infectious disease, endocrinological, neurological, ophthalmological, nephro-urological, and psychiatric conditions.
Both groups in the study were compared in terms of several variables, including the following: arthropathy, psoriasis, biological drug naïveté at the beginning of treatment, biologic type, Psoriasis Area Severity Index (PASI) at T0, 16 weeks, 28 weeks and 52 weeks and PASI-100, PASI-90 and PASI<3 at the point of 16, 28 and 52 weeks.
For dichotomous continuous variables, the investigators utilized nonparametric tests, and they used Fisher and chi-square tests when they were needed (relative to the amount of involved patients).
Among the patients included in the study, the most frequent comorbidities were found the research team to be hypertension in 105 patients, type 2 diabetes mellitus in 32, dyslipidemia in 26, acute ischemic cardiopathy in 25, COPD in 13, symptomatic benign prostatic hyperplasia in 19, arthrosis in 11, HCV in 9, asthma in 5. Eighty-one were found to have had other comorbidities.
Both the comorbidity and the non-comorbidity groups were noted by the investigators as not differing in terms of BMI (P = 0.581), associated arthropathy (P = 0.744), sex (P = 0.951), difficult-to-treat regions affected by psoriasis (P = 0.114), or treatment-naïve status for biological drugs at the start of treatment (P = 0.419).
The investigators analyzed the use of 6 drugs for psoriasis, patients with comorbidities, secukinumab was the most frequently administered drug at 26.03%, followed by risankizumab at 21.46%, and brodalumab at 20.09%. In those without any reported comorbidities, the team reported that ixekizumab was the drug prescribed most often at 28.33%, followed by tildrakizumab at 25%, and secukinumab also at 25%.
Overall, the research team found no statistically significant difference in the frequency of drugs administered to the patients. Despite this fact, more patients with comorbidities achieved PASI-90 and PASI-100 at 28 weeks than those without comorbidities (56.65% vs. 36.96% and 65.32% vs. 43.48%, P = 0.007, P = 0.02, respectively).
They added that they did not observe a statistically significant correlation between the 2 groups for PASI scores at 16, 28, 52 weeks or PASI < 3 at 16 and 52 weeks.
“However, it should be noted that the lack of statistical significance in this analysis means that while no impact was observed, it is not possible to definitively conclude that there was no impact at all,” they wrote.
References
- Rosset F, Mastorino L, Dapavo P, Quaglino P, Ribero S. Impact of comorbidities in elderly and frail patients and response to biological therapy in psoriasis. Exp Dermatol. 2023 Apr 26. doi: 10.1111/exd.14822. Epub ahead of print. PMID: 37098693.
- Simpson EL, Irvine AD, Eichenfield LF, Friedlander SF. Update on epidemiology, diagnosis, and disease course of atopic dermatitis. Semin Cutan Med Surg. 2016;35:84S-88S.
- Ruggiero A, Fabbrocini G, Cinelli E, Ocampo Garza SS, Camela E, Megna M. Anti-interleukin-23 for psoriasis in elderly patients: guselkumab, risankizumab and tildrakizumab in real-world practice. Clin Exp Dermatol. 2022;47:561-567.





