Article
Hyporesponsiveness to CKD Therapy Increases RBCTs in Medicare Patients
Author(s):
Hyporesponse to erythropoiesis-stimulating agents was associated with increased rates of red blood cell transfusions and poorer outcomes in patients with chronic kidney disease, highlighting the need for alternative anemia treatments.
Christine Ferro, Milliman
Credit: Milliman, Inc.

Results from a poster presentation at National Kidney Foundation Spring Clinical Meetings 2023 show an increased use of red blood cell transfusions (RBCTs) in patients with persistent hyporesponse to erythropoiesis-stimulating agents (ESAs).1
Akebia Therapeutics shared the research along with findings from another investigation on chronic kidney disease.2
Patients with hyporesponse to ESAs demonstrate an inadequate response to the treatment, resulting in anemia and low red blood cell count. This condition has been linked with adverse outcomes and events, according to the investigation.
These patients also incurred expensive healthcare costs, indicating the need for alternative treatments for anemia. Additional data from the study revealed that patients receiving peritoneal dialysis had the lowest rates of RBCTs and the highest rates of kidney transplantations, regardless of their hyporesponse status.
Christine Ferro, of Milliman, and a team of investigators sought to evaluate disparities in RBCTs and kidney transplantation rates between Medicare patients with and without hyporesponse to ESAs.
Erythropoiesis-Stimulating Agents and Healthcare Utilization
The study was conducted using data from 100% Medicare fee-for-service beneficiaries who were receiving dialysis for chronic kidney disease and were using ESAs for at least 6 months.
Patients were classified as hyporesponive if they had 2 consecutive months (≥60%) with a mean ESA weekly dose of >300 epoetin alfa units per kg of body weight and a hemoglobin level in the prior month that was <10 g/dL. Patients with these levels for <60% of the time frame were considered to have an acute hyporesponse.
Investigators observed the mean days with RBCT procedures and the risk-normalized kidney transplantations per patient, per year. Then, the team stratified patients based on their dialysis modality to assess disparities.
Frequency of Transfusions in Hyporesponsive Patients
The population consisted of more than 2 million patients, and 21,302 (10.3%) patients were defined as hyporesponsive to ESAs based according to the study criteria. After analysis, the distribution of patients by dialysis modality was 92% hemodialysis, 6% peritoneal dialysis, and then 2% who received both peritoneal and hemodialysis.
Investigators observed a prominent difference in the average number of days patients participated in RBCTs when examining the group with acute hyporesponse and the persistent hyporesponse group.
Among patients who received hemodialysis, the mean number of transfusion days was almost 12 times higher in those with persistent hyporesponse (3.28) when compared with those without hyporesponse (0.26).
The values in the table represent the mean days with RBCTs for each patient group and dialysis modality combination.
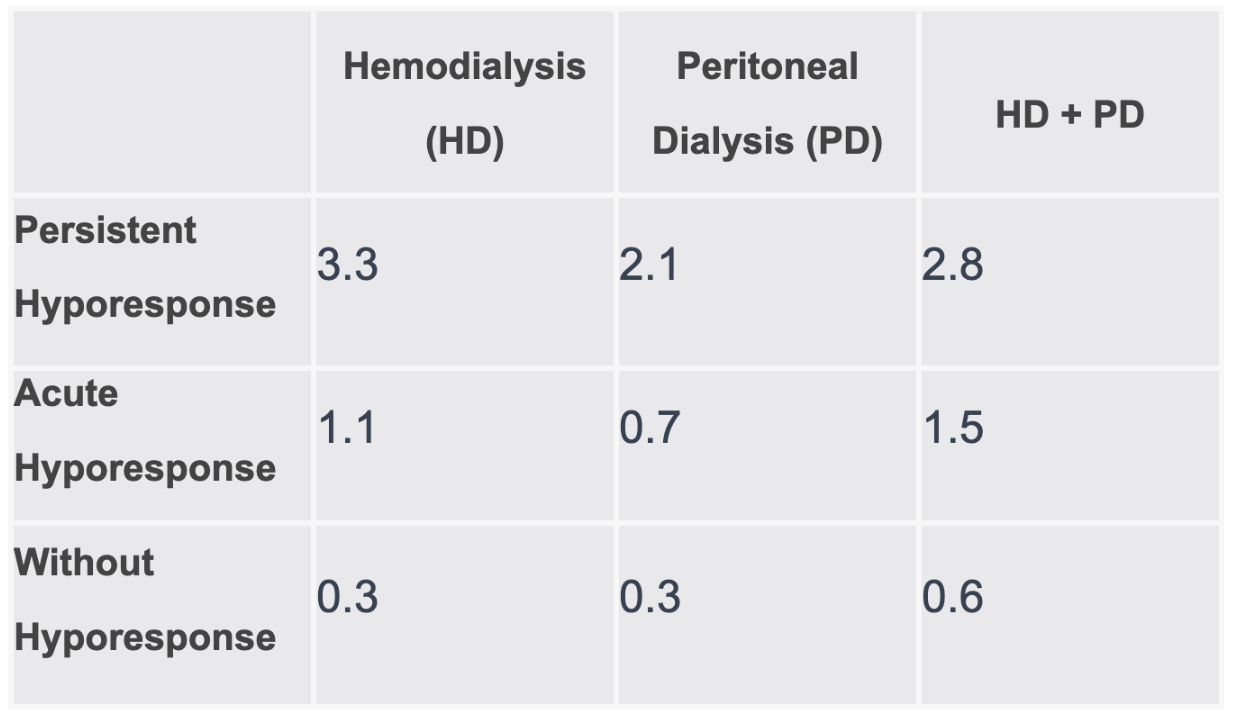
This number was continuously higher for patients with persistent hyporesponse across dialysis modalities. The data exhibited a mean of 3.3 days for hemodialysis, 3.1 for peritoneal dialysis, and 2.8 for both modalities, within this group.
Findings also indicated the lowest rates of RBCTs were consistent with patients who had peritoneal dialysis, as well as the highest risk-normalized rates of kidney transplantation per patient, per year, across all levels of hyporesponse.
This investigation brought attention to the necessity of alternative treatments for patients with hyporesponse, and underlined the importance of considering the dialysis modality when managing anemia in patients with chronic kidney disease, investigators stated. Overall, these findings can provide important insights into the impact of hyporesponse on healthcare utilization and outcomes in this patient population.
References:
- Ferro, Christine. Blood Transfusion and Transplant Rates Among Medicare Patients With Dialysis-Dependent CKD and Hyporesponse to ESAs. Paper presented at: National Kidney Foundation (NKF) Spring Clinical Meetings 2023 (SCM23); April 11-15, 2023; Austin, TX. Accessed April 10, 2023.
- Akebia Therapeutics Announces Poster Presentations at National Kidney Foundation Spring Clinical Meetings 2023. News Release. Akebia Therapeutics. April 7, 2023. Accessed April 10, 2023. https://ir.akebia.com/news-releases/news-release-details/akebia-therapeutics-announces-poster-presentations-national-0





