Publication
Article
Internal Medicine World Report
International Guidelines for Psoriatic Arthritis Are Based on Weak Evidence
Author(s):
Many studies that inform current international guidelines for the treatment of psoriatic arthritis (PsA) are of poor quality and lack applicability in clinical practice.
Many studies that inform current international guidelines for the treatment of psoriatic arthritis (PsA) are of poor quality and lack applicability in clinical practice, according to an analysis published in the August 2014 issue of the Journal of the European Academy of Dermatology and Venereology.
For the review, a team of researchers in France examined the quality of American and European guidelines that concern PsA care (Table 1). While performing their systematic literature search, the investigators excluded studies that inspected biological therapies, as they noted “PsA treatment has rapidly changed over the lasted decade with the important development of biologics, (but) before starting this treatment, non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticosteroids, and disease modifying antirheumatics drugs (DMARDs) are commonly used.”
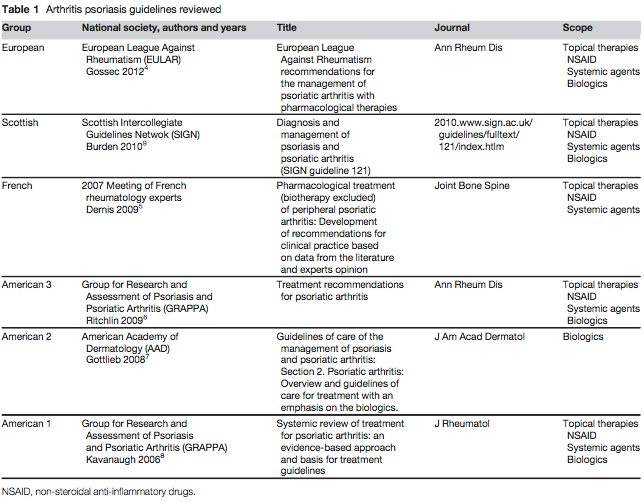
Among the 6 selected guidelines, which had been published since 2007, the researchers found “considerable variation in the quality … according to the Appraisal of Guidelines for Research and Evaluation (AGREE) instrument domains” of scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial dependence.
For instance, the guidelines’ scores ranged from 36-83.3% on scope and purpose and 58.3-85.7% on rigor of development, “particularly because of inadequate discussion of procedure for updating,” the analysis authors wrote. Although the guidelines’ range of AGREE scores on editorial independence and clarity of presentation were less problematic, “the lowest domain scores were observed in applicability because of inadequate discussion of all items, particularly of cost implications.”
As a result, the researchers concluded that, “although their rigor of development is of good quality, many of the studies that (the guidelines) included are of poorer quality.”
“All the guidelines recommend an NSAID as first-line treatment in patients when it is not contraindicated, although the data regarding the usefulness of NSAIDs in PsA are limited. Similarly, there is no evidence from clinical trials on the efficacy of intra-articular or systemic corticosteroids in PsA, (and) regarding DMARDs, although many of them are widely used, they do not have a strong published evidence base,” the investigators wrote. “Indeed, there are few evidence-based studies on the treatment of PsA.”
Despite the obvious need for more controlled, high-quality trials on the non-biological treatment of PsA to improve the current guidelines, the researchers relented that such studies are “unlikely to be conducted, given the lack of interest in studying old drugs.” In fact, only one such randomized controlled trial has been published since the release of the most recent PsA treatment guideline, they noted.






