Managing degenerative lumbar spinal stenosis
This article describes the diagnostic evaluation of degenerative lumbar spinal stenosis, approaches to nonsurgical treatment, indications for surgery and principles of surgical management.
Lumbar spinal stenosis is a narrowing of the vertebral canal, lateral recess, or intervertebral foramina of the lumbar spine; the condition may be congenital or result from spinal degeneration.1 Usually, degenerative or acquired spinal stenosis does not become symptomatic until patients reach middle age. It affects men nearly twice as often as women.
Typical symptoms of spinal stenosis include pain, numbness, and paresthesias in the posterolateral thighs and legs that radiate distally in a dermatomal distribution. Patients may also complain of weakness or "heaviness" in the lower extremities. Classically, these symptoms are aggravated by prolonged walking or standing and relieved with sitting. Activities in which the lumbar spine is in flexion, such as walking uphill, leaning forward on a walker or shopping cart, or riding a bicycle, are better tolerated by patients who have neurogenic claudication caused by spinal stenosis.
Usually, patients with spinal stenosis seek medical attention when their quality of life diminishes. Surgical intervention is not always needed. Physicians who gain an understanding of the pathomechanics of the disease, do an appropriate workup, and provide nonoperative treatment can afford many patients their best opportunity to enjoy maximum function.
In this article, we describe the diagnostic evaluation of degenerative lumbar spinal stenosis and approaches to nonsurgical treatment. We also discuss the indications for surgery and principles of surgical management.
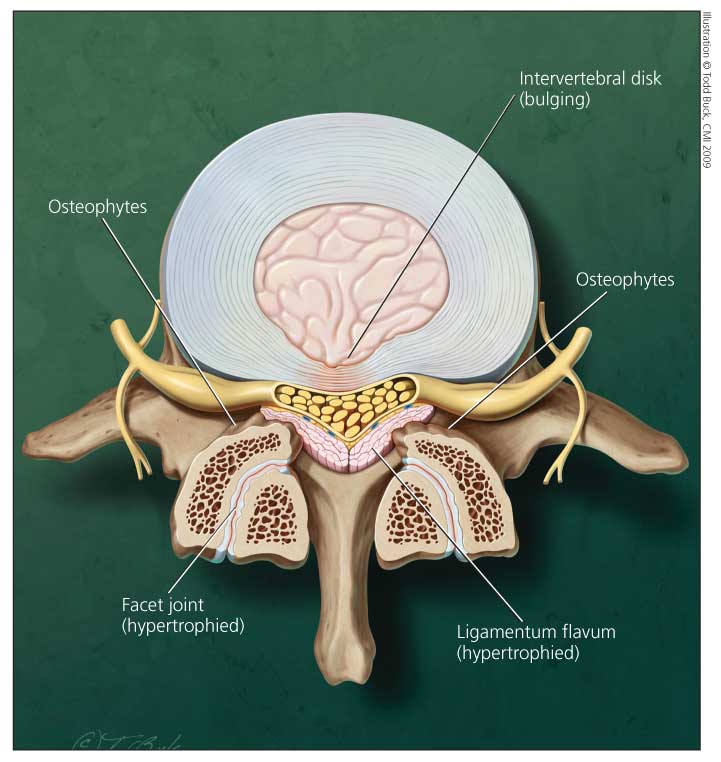
Figure 1 – In a patient with lumbar spinal stenosis, intrusion of the intervertebral
disk anteriorly and of the hypertrophied facet joint and ligamentum flavum
posterolaterally cause the characteristic "trefoil" appearance as seen in this
cross-sectional view of the spinal canal.
DIAGNOSISCauses and anatomy
In degenerative lumbar spinal stenosis, enlargement of osteoarthritic facet joints may result in medial encroachment on the spinal canal. A congenitally narrow canal may limit the ability to tolerate minor acquired encroachment. Soft tissues, such as a hypertrophied ligamentum flavum or herniated disk, also may contribute significantly to thecal sac compression (Figure 1). Frequently, degenerative spondylolisthesis (most commonly at L4-5) causes L5 radiculopathy resulting from root compression in the lateral recess between the hypertrophied subluxated inferior facet of L4 and the posterosuperior aspect of the body of L5.
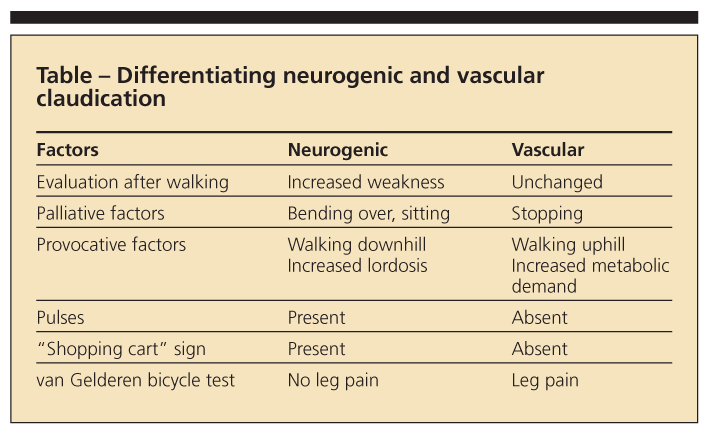
Differentiating between neurogenic and vascular claudication is critical in confirming the diagnosis of spinal stenosis (Table).2 Typically, vascular claudication is associated with cramping calf pain that radiates proximally. In peripheral vascular disease, the symptoms are aggravated by activity (eg, walking a specific distance) in any position and relieved by rest in any position.
The physical examination also is useful for ruling out other conditions that limit walking tolerance, such as vascular claudication. If pulses are absent or asymmetrically diminished, consultation with a vascular surgeon is indicated. In patients who have symptomatic lumbar spinal stenosis, however, often there is little to find on physical examination. A slightly forward flexed position may be noted with standing or walking. Although this approach is not always practical, patients are best examined after having walked until
symptomatic. Patients with neurogenic claudicant leg pain can tolerate bicycle riding (van Gelderen bicycle test) because the flexed position is required. Osteoarthritis of the hips needs to be excluded by evaluating range of motion of the hip joints. Peripheral neuropathy tends to be less painful and more sensory- and weakness-oriented than spinal stenosis.
Imaging
When lumbar spinal stenosis is suspected clinically, the diagnosis should be confirmed with imaging studies. Weight-bearing plain radiographs-including anteroposterior (AP) and flexion/extension lateral views-of the lumbar spine should be used as the initial screening tool, although they are not diagnostic for spinal stenosis. In this patient population, radiographs usually reveal only degenerative changes, such as loss of intervertebral disk height and facet arthrosis. However, they also may provide evidence of spondylolisthesis or scoliosis that may affect surgical decision making. Plain radiographs also are important in helping physicians identify other spinal pathology (eg, compression fracture, neoplasia, or infection) that warrants immediate referral to a spine surgeon.
MRI is the imaging study of choice for identifying the presence of neural compression. MRI not only provides excellent anatomical detail but also clearly depicts the extent of stenosis and identifies the levels that are involved. In particular, T2-weighted sagittal and axial images are best for evaluating the extent of central canal and lateral recess stenosis; T1-weighted parasagittal images usually are best for evaluating neuroforaminal stenosis. MRI should be performed when spinal stenosis is suspected by history and physical examination and the patient has reached a stage of functional impairment (decreased walking tolerance) that he or she can no longer tolerate.
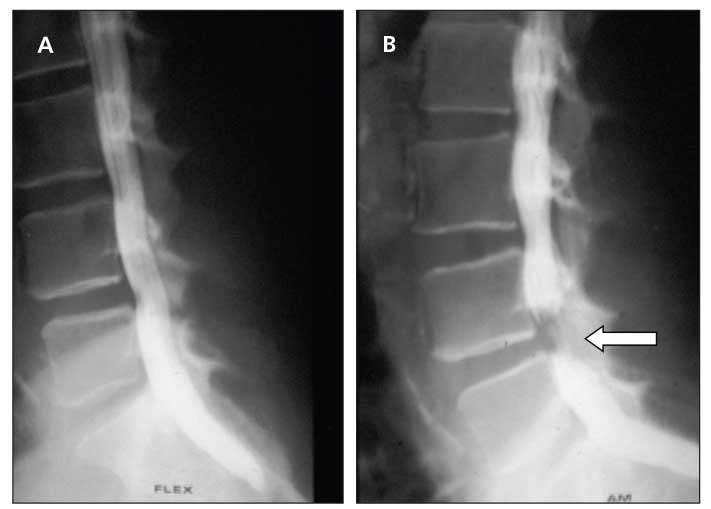
Figure 2 – In a patient with neurogenic claudication, dynamic lateral myelograms of the lumbar spine with flexion (A) and extension (B) reveal significant cutoff at the L4-5 level (arrow) in extension only.
Myelography and postmyelography CT are other imaging modalities that can provide additional information about the extent of stenosis (Figure 2). They are the studies of choice when an MRI cannot be obtained (eg, a patient has a pacemaker or spinal stimulator). Lumbar myelography and postmyelography CT are extremely beneficial when degenerative scoliosis is also present. Coronal plane deformity complicates interpretation of both the axial and sagittal MRI slices. In addition, CT is better for looking at bony detail and compression from osteophytes. The findings from these images can help physicians make surgical planning decisions, particularly for patients who have concomitant degenerative scoliosis.
TREATMENT
Once the diagnosis of degenerative lumbar spinal stenosis is established clinically and confirmed radiographically, treatment should commence. Options for low back pain and neurogenic claudication related to lumbar spinal stenosis include nonoperative modalities, interspinous process spacers (indirect decompression), surgical decompression alone, and surgical decompression and fusion with or without instrumentation. The choice of treatment approach is guided by the severity and duration of symptoms, type and extent of stenosis, associated instability or deformity, degree of disk degeneration, and presence of coexisting medical conditions.
Nonsurgical treatment
A course of conservative treatment is recommended for patients who have mild to moderate neurogenic claudication because it often alleviates symptoms or halts their progression.3 Typically, the onset of lumbar spinal stenosis is insidious and functional loss is slow; therefore, an initial trial of nonsurgical therapy should be attempted. Even in severely stenotic patients, delaying surgery for a trial of nonsurgical treatment presents minimal risks or detrimental effects on surgical outcome.4 If leg pain is present for longer than a year, however, the results of surgical decompression may be poor.5 For most patients with lumbar stenosis, a comprehensive nonsurgical program that includes active patient participation is recommended as the initial treatment.
Passive treatment phase. The first stage of nonoperative treatment involves passive modalities; the goal is pain reduction. Activity modification and relative rest are important in decreasing the severity of symptoms. Bed rest is no longer advocated; now patients are encouraged to become active as soon as they can.
Patients are encouraged to avoid aggravating activities, such as heavy lifting and excessive trunk extension, that decrease the AP diameter of the spinal canal.6 Similarly, use of rigid braces is discouraged; the braces can extend the lumbar spine and aggravate stenosis symptoms, proving to be more harmful than helpful.
Wearing an elastic lumbar binder may provide benefit by reducing loads across the lumbar spine. If a lumbar binder is recommended, it should be worn only for brief periods to avoid deconditioning of the paraspinal musculature.
Use of oral medications, particularly NSAIDs, is recommended initially. Tricyclic antidepressants are effective in some patients with chronic numbness and dysesthetic nerve pain. Oral corticosteroids can reduce nerve root irritation and may be beneficial in acute flares but should be used only briefly, because long-term use has potential adverse effects.7
Muscle relaxants may be prescribed for the relief of muscle spasms, but the patient should be alerted to the potential for addiction. Narcotic medications, which are constipating and potentially habit-forming, should be prescribed sparingly; patients who have incapacitating pain and cannot tolerate anti-inflammatory agents can use them but should do so only for brief periods.
Other nonoperative treatment modalities include cryotherapy, hot packs, manual therapy (massage), acupuncture, transcutaneous electrical nerve stimulation, ultrasonography, traction, and chiropractic therapy. However, no prospective, randomized studies have proved their benefit.
Chiropractic manipulation is most effective in patients who have posture-dependent symptoms, no evidence of segmental instability, and sufficient cognitive and physical abilities to participate. In a case report of multilevel stenosis, flexion-distraction manipulation resulted in a decrease in intensity and frequency of leg pain and even the resolution of back pain.8 It has been speculated that spinal manipulation exerts a powerful placebo effect in producing a specific but short-term benefit.9 It is also suggested that manipulative therapy results in a manual decompression by reducing local ischemia and mechanical compression to chronically irritated nerve roots. We do not recommend chiropractic treatment to all of our patients; if they seek it, we caution against extension manipulation.
Although controversial, use of epidural corticosteroid injections can help reduce the radicular pain associated with acute exacerbations of neurogenic claudication. The injections have analgesic and local anti-inflammatory effects that reduce spinal stiffness and can facilitate eventual progression to the active phase of therapy.10,11 However, in a study limited to patients with symptomatic lumbar stenosis, Hoogmartens and Morelle12 reported that fewer than half of the patients treated with epidural corticosteroid injections demonstrated functional improvement about 2 years after treatment; the outcomes were only slightly better than those with placebo.
In a randomized, prospective, double-blind study, Cuckler and associates13 were not able to ascertain a benefit from interlaminar injection of methylprednisolone acetate compared with physiologic saline at 24-hour and approximately 20-month follow-up. This study was somewhat limited by the authors' definition of 75% or greater subjective improvement over preinjection symptoms as a successful result and their failure to look at outcomes in the interval between a day and a year after injection. Interlaminar and caudal injections once were in common use and still may be best for multilevel pathology.
Although the interlaminar and caudal routes of epidural injection are technically easier than injection through arthritic posterior elements in older patients with spinal stenosis,14 acute radicular-type pain in a specific nerve root distribution is best managed with a transforaminal selective nerve root injection (corticosteroid and bupivacaine) performed under fluoroscopic guidance. Selective nerve root blocks (SNRBs) have provided better outcomes in patients with radicular pain resulting from herniated nucleus pulposus. In one series, however, 72% of patients with stenosis experienced short-term benefit from injection, but only 28% had long-term success.15
In a prospective, randomized, controlled, double-blind study conducted by Riew and associates,16 71% of patients who initially requested surgical intervention decided against surgery after receiving selective nerve root injection with bupivacaine and betamethasone. Because many patients for whom other nonsurgical treatment methods have not succeeded may decide against surgery after an SNRB, patients whose stenosis symptoms have a lumbar radicular pain component should be considered for an SNRB. In addition, an SNRB is a good prognosticator of surgical outcome: patients who obtain greater than 50% relief of lower extremity pain for at least 1 week tend to have 50% or more relief in leg pain (compared with the preoperative intensity) within 1 month after surgery and lasting at least 6 months.5
Derby and coworkers5 also demonstrated a strong correlation among the preoperative duration of leg pain, response to an SNRB, and relief of leg pain after surgical decompression. In this series, all patients who had leg pain for less than a year and a good response to an SNRB had a successful outcome from surgical decompression; 95% of patients who had leg pain for longer than a year and no response to an SNRB had a poor surgical outcome.
Active treatment phase. This phase of nonsurgical treatment consists of formal functional physical therapy, which lays the foundation for faster recovery and decreased disability and pain.17 Programs should be tailored to individual patients to account for any limitations imposed by coexisting medical conditions.
Flexion-based exercises are the mainstay of physical therapy for patients with lumbar stenosis.18 Flexion of the spine dynamically increases the cross-sectional area of the spinal canal; extension dynamically decreases it.19 As a result of increased AP diameter of the spinal canal in flexion, space for the neural elements is increased, microcirculation is improved, and patients can better tolerate the exercise program.6 Both stationary bicycle and inclined treadmill conditioning exercises are effective in patients with lumbar stenosis.17
Exercise is encouraged because it leads to weight loss, improved cardiovascular fitness, and release of endorphins. Aquatic therapy; strengthening of abdominal and trunk musculature; and stretching of hip flexors, hamstrings, and paraspinal muscles also may provide benefits.
Patient education should include training in posture and activities of daily living. Patient education should be a part of every patient's treatment regimen.
We prefer to treat symptomatic patients with one SNRB and monitor their progress. When substantial relief is achieved, physical therapy is instituted and observation is continued. In patients who experience only partial relief of symptoms, we typically recommend a second SNRB 2 to 3 weeks after the initial injection and follow it with functional therapy.
We are not likely to recommend further injection in patients who have no or extremely short-lived pain relief unless we suspect that another area of the lumbar spine should be targeted. If no initial treatment is beneficial, the patient can choose to "live with" the problem or pursue more aggressive surgical options.
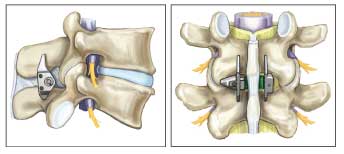
Figure 3 – The X STOP, an interspinous process spacer, can be implanted to prevent extension at the symptomatic level and minimize symptoms of lumbar spinal stenosis. Note that no bone removal is required and anatomy is preserved.
Nonsurgical treatment outcomes
Nonsurgical treatment can minimize the progression of lumbar stenosis symptoms, but it is unlikely to affect the underlying pathoanatomy.20 In a study of the natural course of 32 patients with spinal stenosis who were treated nonoperatively for 4 years, the pain level was unchanged in 70%, improved in 15%, and worsened in 15%.21 Therefore, the authors recommended expectant observation as an alternative to surgery.
In another series, 52% of patients who were treated nonoperatively reported improvement in their predominant pain (back or leg), and 42% were satisfied with the outcome.22 Onel and coworkers23 reported the best results from an aggressive nonoperative treatment regimen: 70% of patients showed significant symptomatic improvement and 23% improved mildly. However, they did not report on the length of follow-up or long-term outcomes.
In patients who show improvement with nonsurgical treatment, it is unclear which components of therapy actually provide a benefit. The decision to provide conservative care is further complicated by an abundance of treatment strategies and a scarcity of modalities that have been subjected to repeated or well-done prospective, randomized studies.
In most patients who are treated nonsurgically, symptoms remain stable over time.21 Although some physicians have found conservative measures to be of little long-term benefit, most recommend nonsurgical therapy as the first line of treatment.
Indications for surgical intervention
Surgical referral is reserved for patients who have intolerable pain, progressive neurological deficit or, rarely, cauda equina syndrome and for those in whom conservative measures have not succeeded. Before a surgical approach is decided on, a thorough medical workup is needed to assess the risks and ensure that the patient can tolerate both the procedure and postoperative rehabilitation. For a discussion of the surgical options and outcomes, see the Box, "Principles of surgical management" (left).
Patient selection in lumbar stenosis surgery is crucial to enhancing outcome. Patients who are functionally limited in both walking tolerance and activities of daily living are potential candidates, but the ultimate decision to proceed with surgery should be made by the patient.24 Intractable pain, especially neurogenic claudication (leg or buttock pain) that has not responded to nonsurgical treatment, is another reason to consider referral to a spine surgeon. However, isolated back pain is not the best indication for surgery; the surgical outcomes of intervention for this symptom are unpredictable.25
Most patients who have spinal stenosis can be treated on an elective basis. Urgent surgical decompression is recommended only in cases in which there is rapidly progressive neurological deficit or cauda equina syndrome (bladder and bowel dysfunction) or both. The ideal surgical candidate would present with severe leg symptoms of a neurogenic claudicatory nature and corresponding stenosis on imaging studies, no or minimal axial back pain, no or minimal neurological deficit, no evidence of vascular claudication, and no medical comorbidities.26
References:
References1. Arnoldi CC, Brodsky AE, Cauchoix J, et al. Lumbar spinal stenosis and nerve root entrapment syndromes: definition and classification. Clin Orthop Relat Res. 1976;115:4-5.
2. Hawkes CH, Roberts GM. Neurogenic and vascular claudication. J Neurol Sci. 1978;38:337-345.
3. Benoist M. The natural history of lumbar degenerative spinal stenosis. Joint Bone Spine. 2002;69:450-457.
4. Amundsen T, Weber H, Nordal HJ, et al. Lumbar spinal stenosis: conservative or surgical management? A prospective 10-year study. Spine. 2000;25:1424-1436.
5. Derby R, Kine G, Saal JA, et al. Response to steroid and duration of radicular pain as predictors of surgical outcome. Spine. 1992;17(6 suppl):S176-S183.
6. Sikorski JM. A rationalized approach to physiotherapy for low-back pain. Spine. 1985;10:571-579.
7. Hilibrand AS, Rand N. Degenerative lumbar stenosis: diagnosis and management. J Am Acad Orthop Surg. 1999;7:239-249.
8. Snow GJ. Chiropractic management of a patient with lumbar spinal stenosis. J Manipulative Physiol Ther. 2001;24:300-304.
9. Curtis P. Spinal manipulation: does it work? Occup Med. 1988;3:31-44.
10. Benzon HT. Epidural steroid injections for low back pain and lumbosacral radiculopathy. Pain. 1986;24:277-295.
11. Rademeyer I. Manual therapy for lumbar spinal stenosis: a comprehensive physical therapy approach. Phys Med Rehabil Clin N Am. 2003;14:103-110, vii.
12. Hoogmartens M, Morelle P. Epidural injection in the treatment of spinal stenosis. Acta Orthop Belg. 1987;53:409-411.
13. Cuckler JM, Bernini PA, Wiesel SW, et al. The use of epidural steroids in the treatment of lumbar radicular pain: a prospective, randomized, double-blind study. J Bone Joint Surg. 1985;67A:63-66.
14. Truumees E, Herkowitz HN. Lumbar spinal stenosis: treatment options. Instr Course Lect. 2001;50:153-161.
15. Schmid G, Vetter S, Göttmann D, Strecker EP. CT-guided epidural/perineural injections in painful disorders of the lumbar spine: short- and extended-term results. Cardiovasc Intervent Radiol. 1999;22:493-498.
16. Riew KD, Yin Y, Gilula L, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain: a prospective, randomized, controlled, double-blind study. J Bone Joint Surg. 2000;82A:1589-1593.
17. Bodack MP, Monteiro M. Therapeutic exercise in the treatment of patients with lumbar spinal stenosis. Clin Orthop Relat Res. 2001;384:144-152.
18. Paine KW. Clinical features of lumbar spinal stenosis. Clin Orthop Relat Res. 1976;115:77-82.
19. Inufusa A, An HS, Lim TH, et al. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine. 1996;21:2412-2420.
20. Zdeblick TA. The treatment of degenerative lumbar disorders: a critical review of the literature. Spine. 1995;20(24 suppl):126S-137S.
21. Johnsson KE, Rosén I, Udén A. The natural course of lumbar spinal stenosis. Clin Orthop. 1992;279:82-86.
22. Atlas SJ, Keller RB, Robson D, et al. Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the Maine lumbar spine study. Spine. 2000;25:556-562.
23. Onel D, Sari H, Dönmez C. Lumbar spinal stenosis: clinical/radiologic therapeutic evaluation in 145 patients: conservative treatment or surgical intervention? Spine. 1993;18:291-298.
24. Sengupta DK, Herkowitz HN. Lumbar spinal stenosis: treatment strategies and indications for surgery. Orthop Clin North Am. 2003;34:281-295.
25. Katz JN, Lipson SJ, Brick GW, et al. Clinical correlates of patient satisfaction after laminectomy for degenerative lumbar spinal stenosis. Spine. 1995;20:1155-1160.
26. Postacchini F. Surgical management of lumbar spinal stenosis. Spine. 1999;24:1043-1047.
27. Hansraj KK, O'Leary PF, Cammisa FP Jr, et al. Decompression, fusion, and instrumentation surgery for complex lumbar spinal stenosis. Clin Orthop Relat Res. 2001;384:18-25.
28. Siddiqui M, Smith FW, Wardlaw D. One-year results of X Stop interspinous implant for the treatment of lumbar spinal stenosis. Spine. 2007;32:1345-1348.
29. Lauryssen C. Appropriate selection of patients with lumbar spinal stenosis for interspinous process decompression with the X STOP device. Neurosurg Focus. 2007;22:E5.
30. Sanderson PL, Wood PL. Surgery for lumbar spinal stenosis in old people. J Bone Joint Surg. 1993;75B:393-397.
31. Ganz JC. Lumbar spinal stenosis: postoperative results in terms of preoperative posture-related pain. J Neurosurg. 1990;72:71-74.
32. Herron LD, Mangelsdorf C. Lumbar spinal stenosis: results of surgical treatment. J Spinal Disord. 1991;4:26-33.
33. Niggemeyer O, Strauss JM, Schulitz KP. Comparison of surgical procedures for degenerative lumbar spinal stenosis: a meta-analysis of the literature from 1975 to 1995. Eur Spine J. 1997;6:423-429.




