Publication
Article
Cardiology Review® Online
Optimal Periprocedural Anticoagulation Strategy in Patients Undergoing Catheter Ablation of Atrial Fibrillation

Suneet Mittal, MD, FACC, FHRS
Review
Lakkireddy D, Reddy YM, Di Biase L, et al. Feasibility and safety of dabigatran versus warfarin for periprocedural
anticoagulation in patients undergoing radiofrequency ablation of atrial fibrillation: results
from a multicenter prospective registry. J Am Coll Cardiol. 2012;59:1168-1174.
Catheter ablation has become accepted therapy for patients with symptomatic atrial fibrillation (AF), which is either refractory or intolerant to at least 1 class I or
class III antiarrhythmic medication.1 However, concerns about the safety of the procedure remain. One of the main concerns is thromboembolism (TE). Patients with AF who are deemed high risk for TE are typically anticoagulated on a chronic basis. Currently approved options in the United States include vitamin K antagonists (eg,warfarin), a direct thrombin inhibitor (eg, dabigatran), or a factor Xa inhibitor (eg,rivaroxaban). An unresolved issue has been how to handle anticoagulation in the peri-AF ablation period.

Patients anticoagulated with warfarin historically have had warfarin discontinued several days prior to AF ablation. Patients were “bridged” with intravenous (IV) or low molecular weight heparin prior to ablation, and then following ablation until their international normalized ratio (INR) became therapeutic. During the ablation itself, anticoagulation was achieved with IV heparin administered to achieve an activated clotting time of 300 to 400 seconds. However, there have been 2 concerns raised with this approach. The first is the risk of TE during the procedure. The transseptal sheath(s) and catheters placed into the left atrium can precipitate thrombus formation, and ablation denudes left atrial endothelium, which may become a nidus for thrombus formation. Heparin alone may be insufficient to prevent thrombus formation. Second, bridging anticoagulation is inconvenient, expensive, and associated with excessive access-site vascular complications, which often necessitate interruption of anticoagulation during a high-risk period (ie, immediately following AF ablation).
These concerns have led investigators to perform the ablation procedure on a continued therapeutic level of anticoagulation. Initial reports suggest that the approach is feasible and avoids the problems inherent to bridging anticoagulation.2 Of note, the 2012 Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society Expert Consensus Statement on catheter and surgical ablation of AF for the first time endorses a strategy of performing AF ablation on continued therapeutic anticoagulation with warfarin.1 Nonetheless, for many operators, concerns remain about the management of patients with procedure-related vascular
complications and/or cardiac tamponade in the face of systemic anticoagulation. The advent of the direct thrombin and factor Xa inhibitors, with their inherent rapid offset and onset of anticoagulation, offers the possibility of getting the “best of both worlds.” Specifically, these agents can be initiated at least 4 weeks prior to ablation, held for 1 to 2 doses prior to the procedure (to allow for reduction in anticoagulation effect), and then reinstituted within the first few hours of the procedure.3 However, data on the efficacy of this approach are lacking in large cohorts of patient undergoing AF ablation.
Study Details
Lakkireddy and colleagues conducted a multicenter, observational study of 290 patients with drug-refractory, symptomatic AF who underwent catheter ablation at 1 of 8 high-volumeAF ablation centers.4 Half (n = 145) of the cohort was treated with dabigatran 150 mg twice daily for at least 30 days prior to the procedure. The medication was withheld the morning of the procedure; a transesophageal echocardiogram (TEE) was performed prior to the ablation procedure to exclude the presence of a left atrial appendage
thrombus. Dabigatran was then restarted 3 hours after hemostasis was achieved. A cohort of 145 patents who were matched for age, sex, and AF type who underwent ablation on therapeutic warfarin (INR 2-3.5) was included as a comparator; no TEE was performed in these patients, all of whom were on therapeutic warfarin for at least 30 days prior to the ablation procedure. The type of ablation procedure performed in the 2 groups did not vary between groups.

The authors found a significantly greater risk of major bleeding in patients treated with dabigatran (6% versus 1% in the warfarin group; P =0.019). In absolute terms, 9 patients in the dabigatran arm had pericardial tamponade, which was the only type of major complication observed in this study. Of concern, in 3 of these patients it presented late (>48 hours after procedure). The authors did not report any difficulty in managing tamponade in these patients, even though an antidote to dabigatran is currently not available. There were no differences in minor complications, such as small groin hematomas or pericardial effusions not requiring drainage. In theory, one could ascribe the excess in major bleeding events to the residual effect of dabigatran (elimination half life, 12 to 14 hours in patients with preserved renal function) since it was withheld only on the morning of the procedure. However, this should then haveprotected patients from an intraprocedural TE. In actuality, the authors observed 3 acute TEs, all in patients with non-paroxysmal AF treated with dabigatran. In multivariate analysis, only age >75 years (odds ratio [OR], 3.82; 95% confidence interval [CI], 1.09-13.35; P = 0.04) and use of dabigatran (OR, 2.76; 95% CI: 1.22-6.25; P = 0.01) predicted the composite end point of bleeding and TE. Thus, on both fronts (minimizing bleeding and TE), dabigatran appeared to perform worse than warfarin.
References
1. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for
patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design.Heart Rhythm. 2012:9:632-696.
2. Di Biase L, Burkhardt JD, Mohanty P, et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: the impact of periprocedural therapeutic international normalized ratio. Circulation. 2010;121:2550-2556.
3. Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. The use of dabigatran immediately after atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2012;23:264-268.
4. Lakkireddy D, Reddy YM, Di Biase L, et al. Feasibility and safety of dabigatran versus warfarin for periprocedural anticoagulation in patients undergoing radiofrequency
ablation of atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2012;59:1168-1174.
Commentary
Dabigatran vs Warfarin in AF Patients Undergoing Ablation
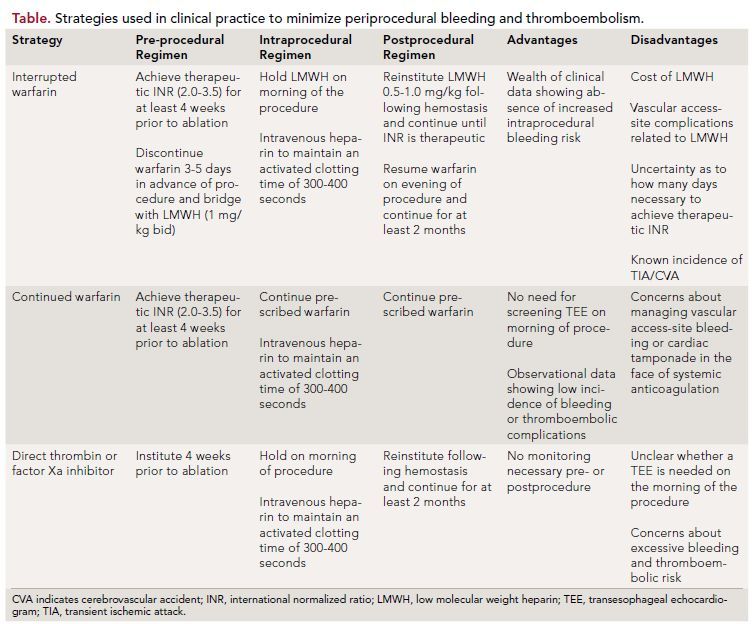
Catheter ablation is increasingly being used to treat patients with AF. Minimizing periprocedural bleeding and TE are major objectives for all physicians performing this procedure. To date, a variety of strategies have been employed in clinical practice (Table); each one is associated with inherent advantages and disadvantages.

Nonetheless, data from Lakkireddy and colleagues appear to provide further support to the notion that ablation during continued therapeutic anticoagulation with warfarin is the optimal strategy in patients undergoing catheter ablation of AF. It should, however, be acknowledged that this was not a randomized study, the differences in TE between the 2 groups may simply have been the result of chance (the difference between the 2 groups was not significant), and that the excess in bleeding may have been minimized by holding dabigatran for 1 to 2 additional doses. From a practical standpoint, a randomized trial evaluating direct thrombin or factor Xa inhibitors to
continued warfarin is desperately needed. Increasingly, patients with AF are being treated with a direct thrombin or factor Xa inhibitor. If it is determined that a patient taking one of these agents requires AF ablation, the electrophysiologist has a real practical challenge. Namely, warfarin will have to be instituted, a dose determined that reliably results in a therapeutic INR, and only then can the ablation procedure be scheduled to allow for at least 4 weeks of pre-procedure anticoagulation. Many patients will balk at the suggestion of instituting warfarin and the uncertainty of scheduling a date for their ablation procedure at the time of initial consultation; many electrophysiologists will lack the infrastructure in their practices to accommodate the need to assume the burden of INR monitoring in their patients pre- and postablation. Thus, in the interim, more data like those provided by Lakkireddy and colleagues are needed to guide clinical decision making.
About the Author
Suneet Mittal, MD, FACC, FHRS, is director of electrophysiology at Valley Hospital Health System in Ridgewood, NJ, and associate professor of clinical medicine at Columbia University, New York, NY. He received his MD from Boston University School of Medicine and did an internship and residency at the Hospital of the
University of Pennsylvania (HUP) in Philadelphia, PA, as well as fellowships in cardiology at HUP and in cardiac electrophysiology at The New York Hospital-Cornell University Medical Center. Dr Mittal has written many articles and chapters on electrophysiology and is a frequent participant in national and international symposia, meetings, and scientific sessions.
