Publication
Article
Cardiology Review® Online
Oral Rivaroxaban for the Treatment of Symptomatic Pulmonary Embolism

Alison L. Bailey, MD
Review
The EINSTEIN-PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism.

N Engl J Med. 2012;366:1287-1297.
Pulmonary embolism (PE) is a common disorder that requires prompt diagnosis and long-term treatment to reduce the associated morbidity and mortality. The mortality of untreated PE approaches 30%, with recurrent venous thromboembolism (VTE) being the most common cause of death.1
The incidence of PE has been increasing (from 62.3 to 112.3 per 100,000), likely due to improved diagnostic accuracy with the increased availability of computed tomographic pulmonary angiography. 2 The classical management of PE consists of initial therapy with a parenteral or subcutaneous anticoagulant such as unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or fondaparinux for 5 days overlapping with an oral vitamin K antagonist (VKA) titrated to a goal international normalized ratio (INR) of 2.0 to 3.0.3 The time course of VKA therapy depends on clinical factors such as the inciting event and comorbid conditions, but is generally 3 to 12 months.
There are many challenges with maintenance VKA therapy; thus, there is great clinical interest in more convenient anticoagulants. Rivaroxaban is an oral direct Xa inhibitor that has been studied for the prophylaxis and treatment of deep vein thrombosis (DVT). In the United States, rivaroxaban is approved by the FDA for postoperative
DVT prophylaxis and thromboembolism prophylaxis in atrial fibrillation (AF). The EINSTEIN-PE trial evaluated the use of rivaroxaban alone versus standard therapy in patients with a pulmonary embolus.4
Study Details
EINSTEIN-PE was a randomized, open-label, event-driven noninferiority trial that compared oral rivaroxaban with standard therapy of enoxaparin (LMWH) followed by adjusted-dose VKA in patients diagnosed with acute, symptomatic PE. The primary efficacy outcome was symptomatic recurrent VTE; the principal safety outcome was
major or clinically relevant non-major bleeding. PE with or without DVT must have been objectively confirmed for the patient to be included in this trial. Prior trials of rivaroxaban monotherapy did not include patients with PE (DVT only), so a dose-confirmation phase was included within this trial to confirm therapeutic equality.
Patients were excluded if they had received therapeutic dosing of LMWH, UFH, or fondaparinux for more than 48 hours or more than 1 dose of a VKA before randomization; were treated with thrombectomy, vena cava filter, or fibrinolytic therapy for the current VTE episode; had contraindications to enoxaparin or VKA therapy; had significant renal (CrCl <30 mL/min) or hepatic disease; had evidence of active bleeding or contraindication to anticoagulant therapy because of a high risk of bleeding; had uncontrolled hypertension (>180/110 mm Hg); had anticipated concomitant use of a strong inhibitor of CYP3A4 or a CYP3A4 inducer; or had a life expectancy <3 months.
The treating physician determined the intended duration of anticoagulant treatment for each patient prior to randomization. Randomization was then
stratified according to country and the intended treatment duration (3, 6, or 12 months). Assignment to either rivaroxaban or standard therapy was then performed. The rivaroxaban group received an oral dose of 15 mg twice daily for 3 weeks followed by 20 mg once daily. The standard-therapy group received enoxaparin injections twice daily (at a dosage of 1 mg/kg body weight) in combination with a VKA (either warfarin or acenocoumarol). Enoxaparin was stopped when the INR was >2.0 for more than 2 consecutive days and the patient had received at least 5 days of enoxaparin therapy. The INR goal was 2.0 to 3.0 and was checked at least once per month. The duration of anticoagulation for patients in each group was based on the pre-randomization plan of care. Concomitant use of aspirin (≤100 mg daily), clopidogrel (≤75 mg daily), or
both was allowed if clinically indicated.
Between March 2007 and March 2011, 4832 patients were enrolled at 263 sites and followed for a mean of 260 days. The patients were well matched at baseline, with a mean age of 57 years and an intermediate extent of PE. Onefourth of the patients had a concurrent DVT and the majority of cases were unprovoked VTE (64%). For the standard therapy group, the median duration of enoxaparin therapy was 8 days, and 83% of the patients reached an INR
of 2.0 or greater by the end of enoxaparin therapy. The proportion of time spent with a therapeutic INR (2.0-3.0) was 62.7%, subtherapeutic INR (<2.0) was 15.5%, and supratherapeutic INR (>3.0) was 21.8%. In the rivaroxaban group, adherence to therapy was >80% in the majority of the patients.
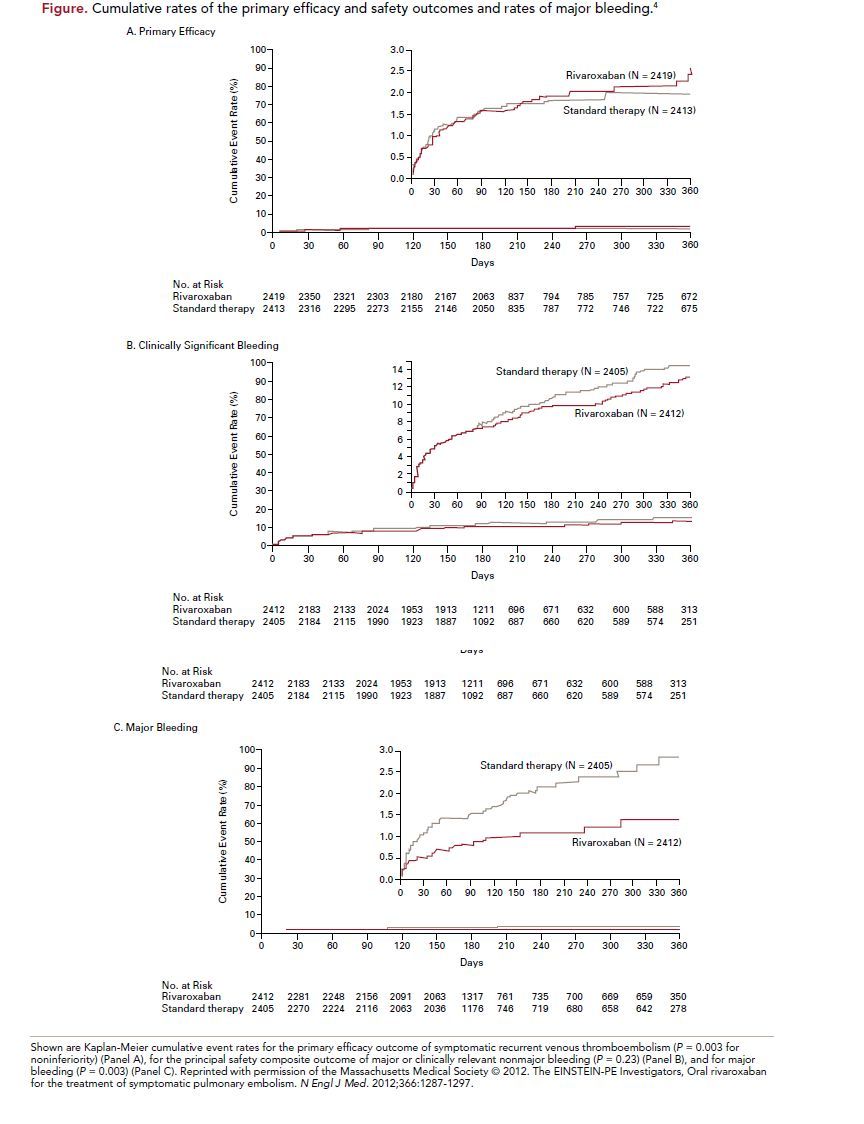
The primary efficacy outcome of recurrent VTE occurred in 2.1% (50 patients) of the rivaroxaban group and 1.8% (44 patients) of the standard therapy group (hazard ratio [HR], 1.12; 95% confidence interval [CI], 0.75-1.68; P = 0.003 for noninferiority, P = 0.57 for superiority) (Figure). The principal safety outcome of first major or clinically relevant non-major bleeding episode occurred in 10.3% (249 patients) of the rivaroxaban group and 11.4% (274 patients) of the stand therapy group (HR, 0.90; 95% CI,
0.76-1.07; P = 0.23). Major bleeding occurred in 1.1% (26 patients) of the rivaroxaban group and 2.2% (52 patients) in the standard therapy group (HR, 0.49;
95% CI, 0.31-0.79, P = 0.003). Similar results were seen across all prespecifiedsubgroups.
In this study of patients with symptomatic PE, a single-drug regimen with oral rivaroxaban was not inferior to standard therapy of enoxaparin and a VKA for efficacy, and may have a better side-effect profile.
References
1. Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326:1240.
2. Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171:831.
3. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease. In: Antithrombotic Therapy and Prevention of Thrombosis. 9th ed. American College
of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141;e419Se494S.
4. The EINSTEIN-PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients. In: Antithrombotic Therapy and Prevention of Thrombosis. 9th
ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl);e278S-e325S.
6. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients. In: Antithrombotic Therapy and Prevention of Thrombosis. 9th ed. American
College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227S-e77S.
7. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients. In: Antithrombotic Therapy and Prevention of Thrombosis. 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2) (suppl):e195S-e226S.
8. The RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342-2352.
9. The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510.
10. The ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487-2498.
11. Van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest. 2006;129;1155-1166.
12. Mueck W, Lensing AW, Agnelli G, Decousus H, Prandoni P, Misselwitz F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50:675-686.
13. Agnelli G, Gallus A, Goldhaber SZ, et al. Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-
DVT (Oral Direct Factor Xa Inhibitor BAY 59-7939 in Patients with Acute Symptomatic Deep-Vein Thrombosis) study. Circulation. 2007;116:180-187.
COMMENTARY
New, Acceptable Options for Anticoagulation of VTEs
V
TE is a commonly encountered clinical problem. Recommendations for both prophylaxis and treatment differ based on baseline risk, clinical scenario, and bleeding risk, and can be quite complex.3,5-7 Until recently, initial parenteral anticoagulation with overlapping VKA therapy was the only option for treatment of VTE. There are many challenges with VKA therapy, including the slow onset of action, difficulty in maintaining therapeutic drug levels, and need for frequent blood monitoring, as well as the increased bleeding risk. Recently, several new oral anticoagulants have been introduced that appear to have acceptable side-effect profiles when used for the prophylaxis and treatment of VTE.8-10 The EINSTEIN-PE trial sought to evaluate the role of oral rivaroxaban monotherapy for symptomatic PE and compare outcomes of both efficacy and safety with the standard of care, LMWH plus a VKA.4
The desire for less cumbersome oral anticoagulants than VKAs has existed for many years. However, until recently there were no viable options. Warfarin is the mostcommonly used VKA in clinical medicine and is a burden to both patients and health care providers. Its slow onset of action and narrow therapeutic window, combined with multiple food and drug interactions, necessitate frequent monitoring of blood levels and dose adjustments. Despite this, maintenance of the drug in a therapeutic range is difficult to achieve. In clinical trials, a therapeutic INR (2.0-3.0) is maintained only about 66% of the time and 57% of the time in clinical practice.11 This is problematic as both insufficient and excessive anticoagulation therapy significantly increases the risk of thrombotic and bleeding events, respectively. In the EINSTEIN-PE study, the INR was within the therapeutic range 62.7% of the time and >3.0 only 15.5% of the time, thus representing a “realworld” treatment group and comparable to therapeutic ranges seen in other contemporary trials. Adherence to rivaroxaban was high and drug discontinuation due to adverse events was low and similar in the rivaroxaban and standard therapy groups (4.6% and 3.8%, respectively). Rivaroxaban was not inferior to standard therapy for recurrent VTE, with a lower rate of major bleeding. The ease of administration and lack of monitoring with rivaroxaban, combined with a potentially improved riskbenefit profile, will make this therapy preferable to warfarin for many patients and health care providers.
This trial enrolled a broad spectrum of patients presenting with hemodynamically stable, symptomatic PE. A moderate proportion had concurrent DVT (25%) and
met the study definition of “extensive” anatomic disease (25%). There was no difference in treatment outcomes of recurrent VTE or bleeding for any of the prespecified subgroups, suggesting no need for either monitoring or dose adjustments in the majority of patients. Patients who met the criteria for thrombolytic therapy were not enrolled in this trial and thus this treatment should not be extrapolated to that group of patients.
The dose of rivaroxaban used in this trial deserves discussion: 15 mg twice daily for the first 3 weeks and then 20 mg daily thereafter. The authors wanted to provide intense anticoagulation initially, as it is known that inadequate anticoagulation for initial therapy of VTE leads to unacceptably high recurrence rates. Additionally, in phase 2 trials, pharmacokinetic data show that earlier steady-state levels, higher trough levels, and better thrombus regression at 3 weeks occurs when using twice-daily versus once-daily dosing of rivaroxaban.12,13 There did not appear to be any early hazard in bleeding risk with this more intensive anticoagulant regimen and, as stated earlier, noninferiority to standard therapy was proven.
One of the biggest clinical concerns with rivaroxaban (and other new anticoagulants) is the lack of a specific antidote or reversal agent. The half-life of rivaroxaban is 5 to 9 hours with a peak plasma level after 2 to 4 hours of drug ingestion. Obviously, cases of acute bleeding (ie, trauma or gastrointestinal hemorrhage) are the most concerning. However, enoxaparin and other LMWHs have been widely used in clinical practice without a specific antidote in existence and have remained the standard of care without overwhelming complications. The biggest potential difference in oral rivaroxaban and LMWH will be the duration of therapy. Anticoagulant therapy for the
treatment of a PE or thromboprophylaxis for AF require much longer time periods than the typical use of LMWH for a few days while bridging to a longer-term therapy.
Whether a clinically significant bleeding risk ensues because of lack of reversibility with this new generation of anticoagulants remains to be seen.
The other concern with rivaroxaban (and other new anticoagulants) is the cost compared with warfarin. These medications generally cost the patient 5 to 100 times as
much as warfarin (depending on presence of insurance and amount of insurance copay). Economic analyses will be important to determine the true cost of warfarin anticoagulation when taking into consideration laboratory monitoring, person-time to adjust doses, medical costs of unintended supra- and sub-anticoagulation, and travel costs to the patient.
In the past few years, multiple new oral anticoagulants have been developed. Most of these drugs focus on directly inhibiting either Factor Xa (rivaroxaban, apixaban,
edoxaban) or thrombin (dabigatran). The benefits of these drugs include ease of administration (oral versus intravenous or subcutaneous), no need for routine monitoring, and improved safety profiles (driven mostly by a reduction in major bleeding episodes). The drawbacks include cost and the irreversible nature of most of these drugs. With currently expanding indications (prophylaxis and treatment of DVT and PE, thromboembolism prophylaxis in AF, acute coronary syndrome therapy) and the lack of enthusiasm for VKAs, these medicines are likely here to stay.
About the Author
Alison L. Bailey, MD, is assistant professor of medicine and director of cardiac rehabilitation at the Gill Heart Institute, Division of Cardiovascular Medicine, at the University of Kentucky in Lexington. She is also associate director of the cardiovascular fellowship program. Dr Bailey received her MD from the University of Kentucky College of Medicine and completed her residency and fellowship at University of Kentucky Chandler Hospital. Her clinical interests include cardiovascular disease in women and cardiovascular disease prevention. Dr Bailey has been published in numerous peer-reviewed medical journals.






