Shared Decision Making for Early Arthritis: The Physician's Role
When and how choices were made and altered help determine the approach totreatment. Becoming an informed consumer helps a patient take control.
ABSTRACT: Early diagnosis and disease-modifying antirheumatic drugs are needed for optimal disease control in patients with undifferentiated inflammatory arthritis or rheumatoid arthritis.When and how choices were made and altered help determine the approach to treatment. Becoming an informed consumer of medical information helps a patient take control; the primary care physician is responsible for providing information. A few key facts help the physician determine whether the joint problem should be taken seriously and discussed with a rheumatologist,who needs to ensure that there is inflammation and define the pattern of joint involvement. A predictive model has been developed to allow for individualized decisions about initiation of treatment. A care contract between physicians and patient may ensure the best outcomes. (J Musculoskel Med. 2008;25:365-374)
Helping a patient who has undifferentiated inflammatory arthritis or rheumatoid arthritis (RA) achieve the best outcome requires the earliest possible correct diagnosis and the use of disease-modifying antirheumatic drugs (DMARDs) for optimal disease control and induction of remission. How to arrive at that desired outcome varies according to the "aggressive personality" of the arthritis and when and how therapeutic choices were made and altered, as guided by the responsiveness of the disease.1,2
As with all illnesses, the patient who has inflammatory arthritis or RA and his or her family are stunned by the realization that he has moved from a state of complete health to ill health, and they enter an information fact-finding mode, regardless of the severity of the problem or the probability of it becoming a lifelong illness. The current sources of medical information include the primary care physician, the Internet, friends and family, and the local bookstore.
Becoming an informed consumer of medical information often delivers a patient from feeling controlled by the illness to being in control. As such, a part of the primary care physician's responsibility is to provide information, lighten the patient's emotional load,and allow him to come to grips with the reality of his medical problem as well as take charge of his health proactively. It also is crucial that the primary care physician, rheumatologist, and patient form a triad of care in which they agree to a co-management contract to ensure that none of the patient's arthritis or general medical issues "falls through the cracks."
In this article, I discuss patient concerns-in the form of questions and answers-about the diagnostic, therapeutic, emotional, and functional aspects of having arthritis and, specifically, RA.The goal is to help define the primary care physician's role in shared decision making to achieve optimal outcomes.
1. What is early arthritis? When should the patient and the primary care physician be concerned that this may not be a common, self-limited musculoskeletal problem?
Arthritis may be defined as joint inflammation. To the patient and primary care physician, this means joint pain, stiffness, swelling, redness, and dysfunction. Given the heightened activity level in most persons' lives in recent years, these symptoms and signs could simply be related to a repetitive overuse syndrome or mild trauma to a joint in a "weekend warrior" involved in sports activity or exercise or to performance of a mundane activity, such as using a computer.
A few key facts help the physician determine whether the joint problem should be taken seriously and discussed with a rheumatologist or simply reflects a self-limited disorder. The facts include the following:
• Pain, swelling, redness, tenderness, stiffness, and limited range of motion in 1 or more joints for 4 or more weeks, particularly if those joints include the wrists, metacarpophalangeal or proximal interphalangeal joints of the hand, or similar joints in the feet.
• Joint inflammation accompanied by functional limitation, morning stiffness lasting longer than 30 minutes, and fatigue.
• No other obvious cause of the inflammation, such as joint trauma; overuse; a viral illness; or a previously well-defined joint problem, such as osteoarthritis, RA, psoriatic arthritis, or infection.
2. When should the patient be referred to a rheumatologist?
Patients who present with arthritis of more than 1 joint should be referred to and seen by a rheumatologist, ideally within 6 weeks of symptom onset. Rheumatologists are better equipped than primary care physicians to differentiate a "benign"musculoskeletal problem from an inflammatory one. They also can better sort out which type of inflammatory joint disorder is present, what clinical and laboratory assessment is appropriate, and which initial treatment regimen is best.3,4
3. What key clinical facts and laboratory tests can help define the cause of the problem and guide treatment choices?
The rheumatologist needs to ensure that there is inflammation and then define the pattern of joint involvement. In the earliest phases of inflammatory polyarthritis-before a definitive diagnosis can be made-only a few joints may be inflamed, but the persistence of the inflammation is a key fact. The joint examination allows for a numeration of swollen and tender joints and helps characterize the joint pattern to define the cause of the problem (Table).
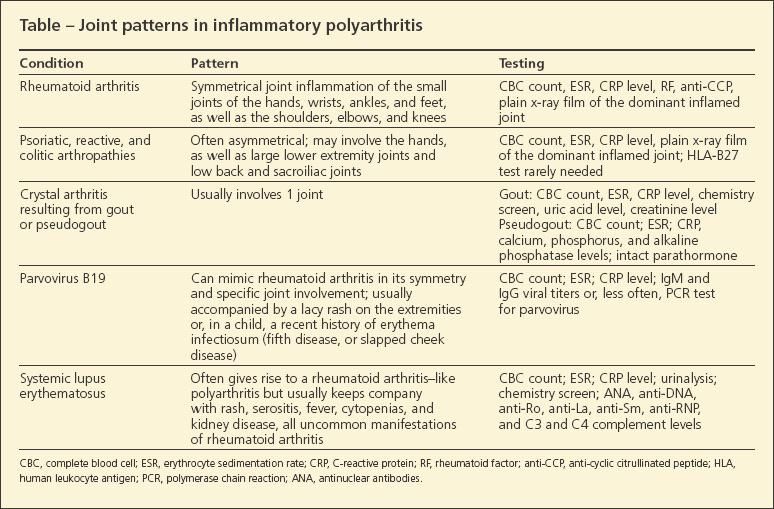
Extra-articular disease manifestations may support one diagnosis or another.Uveitis is common in the spondyloarthropathies; episcleritis and scleritis may be seen in RA. Fever is common in systemic lupus erythematosus (SLE) but not in RA. Characteristic manifestations of the spondyloarthropathies, but not RA, are plantar fasciitis, Achilles tendinitis, low back pain, and sacroiliac pain. Rheumatoid nodules, now a rarity, may be seen in RA but not in the other disorders.
Frequently ordered screening blood tests include a complete blood cell count with differential and platelet counts (anemia and thrombocytosis reflect inflammation), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level (inflammation leads to elevations of both), and rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) (these autoantibodies often are found together in RA but may occur alone; the presence of either one reflects a worse prognosis and poorer outcome and thus demands a more aggressive treatment posture). Anti-CCP–positive and anti-CCP–negative disease seem to be very different illnesses in terms of their severity and genetic background, the former being more aggressive than the latter.
Other screening blood tests include antinuclear antibodies (ANA) (a positive test result could reflect SLE,but some patients with RA may have a weakly positive ANA test result), parvovirus IgM and IgG titers (elevated IgM titers reflect an ongoing infection and the likely cause of RA-like polyarthritis, usually a self-limited, 1- to 3-month illness), hepatitis C serology; and thyroidstimulating hormone. Hashimoto thyroiditis is the most common autoimmune disorder and one of the most common thyroid diseases; hypothyroidism that results from it may lead to aches and pains, carpal tunnel syndrome, and fatigue.
4. Once an inflammatory polyarthritis or early RA has been defined,what are the best treatment options?
Whichever diagnosis of an inflammatory arthritis is made, the earlier that treatment is instituted, the better the outcomes will be. "Tight control" of inflammation-using disease activity scores and health assessment questionnaires, along with single or multiple DMARDs-provides the patient with the best chance for decreased joint damage and a more functional life. Following are some common therapeutic approaches:
• Patient A: Undifferentiated, inflammatory polyarthritis.This patient has 2, 3, or more joints that have been inflamed for 4 or more weeks; negative RF, anti-CCP, and ANA test results; fatigue; an elevated ESR and CRP level; and no erosions on x-ray films. Note that the patient's illness has not risen to the clear ranks of RA, but there is persistent, limiting inflammation and something needs to be done about it.
In Early Arthritis Centers-programs established to promote early identification and management of arthritis and to support research efforts-this type of undifferentiated illness has gone into remission on its own or in the setting of an anti-inflammatory drug regimen in about 25% to 50% of cases, evolved into seropositive RA in about 25% of cases, and persisted as a less aggressive seronegative RA in about 25% of cases.5 Studies have shown that once the illness persists for longer than 3 months, it probably will become chronic.
A reasonable treatment approach for this patient is NSAIDs, short courses of prednisone (20 mg on the first day, tapering by 5 mg over 4 days to 0 mg), and intra-articular corticosteroid injections. If the inflammation persists for another month or so,many physicians would add weekly oral methotrexate (MTX) at a dose of 10 mg, with an escalation to 20 mg in 4 weeks. Randomized studies have shown that the addition of MTX makes the illness becoming a chronic condition like RA much less likely.This outcome supports the concept of a "therapeutic window of opportunity," wherein the illness may be "aborted" by "rebooting" the immune system at a critical point in its evolution.6-9
• Patient B: Early RA. This patient has a symmetrical polyarthritis of the small joints of the hands, wrists, ankles, and feet for 3 to 6 months; positive results of RF or anti-CCP testing or both; fatigue; functional limitation; and an anemia and thrombocytosis. Erosions may be seen on plain x-ray films, but they are rare during the 6 months after disease onset. If erosions are not found, they may be seen on ultrasonography or MRI. Early RA is defined in several ways, varying from joint inflammation for only 6 months to inflammation for less than 2 years.
Most rheumatologists treat this patient with weekly oral MTX as noted above, moving up after 1 month to 20 to 25 mg/wk. NSAIDs and short courses of corticosteroids also are used. Should this patient not improve by 80% within 2 to 3 months, many rheumatologists would add a tumor necrosis factor α (TNF-α) inhibitor. Performance of dynamic exercises guided by a physical therapist and an occupational therapist is important.
An alternative approach that supports the use of combinations of DMARDs involves beginning combination therapy with corticosteroids, MTX, hydroxychloroquine, and sulfasalazine. The key here is to "strike while the iron is hot" and bring this highly inflammatory disorder under control. Early, aggressive treatment ensures a better outcome, and better outcomes may be achieved at any time in the disease course. Recent studies have shown that when treated with a TNF-α inhibitor and weekly MTX, more than 50% of patients who have RA for less than 2 years can go into remission and discontinue the TNF-α inhibitor.10-17
5. Are there clinical facts that can help predict the outcome of early, inflammatory arthritis?
Recently, a predictive model was developed for undifferentiated polyarthritis.18 In this model, age, sex, localization of arthritis symptoms, morning stiffness, tender joint count, swollen joint count, CRP level, RF positivity, and the presence of anti-CCP antibodies are factored into a score. Patients with a higher score are more likely to progress to RA; those with a lower score are less likely. Predicting the risk of RA allows for individualized decisions about initiation of treatment with DMARDs.
6. What are the best methods for monitoring and assessing disease activity and the patient's functional status?
The goal of RA treatment, at any time during its course, is to "bring the illness to its knees" and attain a state of "no evidence of disease," or remission. True remission would mean that the patient has no signs of active inflammation after all medications are stopped; however, that is still an uncommon outcome in RA.Therefore,we aim for no tender or swollen joints or fatigue, full function, and a normal ESR and CRP level while the patient is receiving medications (eg, MTX). The main question is, How do we best assess RA disease activity and functional status similarly to how we address diabetes mellitus (DM) with hemoglobin A1c values, hypercholesterolemia with lipid testing, and hypertension with blood pressure testing?
To ensure a constant state of no inflammation, every 1 to 3 months the physician should assess the number of swollen or tender joints, obtain patient and physician global assessments of disease activity, measure ESR and CRP level, obtain a Disease Activity Score that quantitates these measures, and use a Health Assessment Questionnaire that defines function. Plain x-ray films should be obtained every 6 to 12 months to define whether new erosions or joint-space narrowing has occurred.
Of note, MRI and ultrasonography can pick up erosions 2 years earlier than plain x-ray films. Therefore, if there is a question as to the destructive personality of the illness, these tests can provide early warning signs of a more aggressive illness and a need for increased therapeutic aggressiveness. "Upping the ante" with medications for uncontrolled disease is mandatory.19-21
Joint damage does not exist without joint inflammation.Thus, joint examination looking for redness, warmth, or swelling in a joint is necessary, and their presence demands referral back to the rheumatologist for further assessment and a possible change in treatment.
7. Does a contract among the primary care physician, rheumatologist, and patient ensure the best joint and global health outcomes?
The patient's joint happens to be attached to a body, one that can be affected by the spillover effect of inflammation on blood vessels in the form of premature atherosclerosis, osteoporosis, amyloidosis, and lymphomas. To define who is responsible for what-arthritis care, control of blood pressure, DM treatment, immunizations, cholesterol levels, managing obesity, and avoidance of cigarette smoking-a contract is needed for the primary care physician-rheumatologist-patient triad.The patient's true global health is an integral part of all these components, and each needs to be addressed with equal vigor.
The contract should contain an agreement from all 3 parties to apportion the responsibility for a patient's medical care, as follows:
• Primary care physician: hypertension, atherosclerosis screening/lipid profiles,DM treatment,immunizations, cancer screening.
• Rheumatologist: the arthritis and its extra-articular manifestations, monitoring of drug therapies, imaging technology to define the presence and extent of joint inflammation or damage, and communication with the primary care physician about any significant changes in the patient's rheumatologic status or adverse effects of medication.
• Patient: keeping his medical appointments; maintaining records of laboratory test results, consultation notes, and a current list of his medications; knowing about the potential adverse effects of medications and reporting any problems to his rheumatologist; and stimulating his physicians to communicate.
As in all human interactions, communication is mandatory. All parties should be in constant contact with the others to ensure the best and safest outcome.
References:
References1. O'Dell J. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350:2591-2602.
2. Mitchell KL, Pisetsky DS. Early rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:278-283.
3. Combe B, Landewe R, Lukas C, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2007;66:34-45.
4. Paget S. The European League Against Rheumatism guidelines for early arthritis. Nat Clin Pract Rheumatol. 2007;3:374-375.
5. Bukhari M, Lunt M, Harrison BJ, et al. Rheumatoid factor is the major predictor of increasing severity of radiographic erosions in rheumatoid arthritis: results from the Norfolk Arthritis Register Study, a large inception cohort. Arthritis Rheum. 2002;46:906-912.
6. van Aken J, van Dongen H, le Cessie S, et al. Comparison of long term outcome of patients with rheumatoid arthritis presenting with undifferentiated arthritis or with rheumatoid arthritis: an observational cohort study. Ann Rheum Dis. 2006;65:20-25.
7. Verpoort KN, van Dongen H, Allaart CF, et al. Undifferentiated arthritis-disease course assessed in several inception cohorts. Clin Exp Rheumatol. 2004;22(5 suppl 35):S12-S17.
8. Harrison BJ, Symmons DP, Brennan P, et al. Natural remission in inflammatory polyarthritis: issues of definition and prediction. Br J Rheumatol. 996;11:1096-1100.
9. Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003;48:1771-1774.
10. van Dongen H, van Aken J, Lard LR, et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007;56:1424-1432.
11. Boers M, Verhoeven AC, Markusse HM, et al. Randomized comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997;350:309-318.
12. Mottonen T, Hannonen P, Leirisalo-Repo M, et al; Fin-RACo trial group. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. Lancet. 1999;353:1568-1573.
13. Landewe RB, Boers M, Verhoeven AC, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46:347-356.
14. Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263-269.
15. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2007;146:406-415.
16. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt Study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381-3390.
17. van der Bijl AE, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Infliximab and methotrexate as inductive therapy in patients with early rheumatoid arthritis. Arthritis Rheum. 2007;56:2129-2134.
18. van der Helm-van Mil AH, le Cessie S, van Dongen H, et al. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum. 2007;56:433-440.
19. Pincus T, Sokka T. Quantitative measures and indices to assess rheumatoid arthritis in clinical trials and clinical care. Rheum Dis Clin North Am. 2004;30:725-751.
20. Aletaha D, Ward MM, Machold KP, et al. Remission and active disease in rheumatoid arthritis: determining criteria for disease activity states. Arthritis Rheum. 2005;52:2625-2636.
21. Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5suppl 39):S100-S108.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.




