Publication
Article
Cardiology Review® Online
Apixaban vs Warfarin in Patients With Atrial Fibrillation: Where Does ARISTOTLE Fit In?
Author(s):

Jennifer G. Bekker, PharmD
REVIEW
Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with Aatrial fibrillation (ARISTOTLE). N Engl J Med. 2011;365:981-992.
Last year brought the FDA’s approval of dabigatran for this indication and, more recently, rivaroxaban. The latest investigational anticoagulant drug seek- ing approval for the AF indication is apixiban, an oral direct factor Xa inhib- itor. Here we review the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial.
Anticoagulants are mainstays of had a high risk of bleeding (includ- therapy in patients with atrial ing uncontrolled hypertension, recent fibrillation (AF) for the preven- stroke, or requirement for dual antition of embolic stroke. Until recently, oral options were limited to chronic warfarin. Given the difficulties of controlling patients on vitamin K antago- nists, the cardiology community has ea- gerly awaited the development of newer entities.
Study Details
The ARISTOTLE trial was a randomized, double-blinded study comparing apixaban with warfarin for the preven- tion of stroke or systemic embolism in patients with nonvalvular atrial AF or flutter. In addition to AF or flutter, all patients had at least 1 additional risk factor for stroke, including previous stroke, transient ischemic attack (TIA), systemic embolism, symptomatic heart failure in the last 3 months, left ventricular ejection fraction ≤40%, age ≥75 years, diabetes mellitus, or hypertension.
Patients were excluded if they had another indication for anticoagulation, platelet therapy), or had severe renal or hepatic dysfunction. A total of 18,201 patients were enrolled from December 2006 to April 2010 and were followed for a mean of 1.8 years.
Patients were randomized to receive apixaban 5 mg twice daily or warfarin adjusted to target an international normalized ratio (INR) of 2.0 to 3.0. Pa- tients in the apixaban group who were ≥80 years of age, weighed ≤60 kg, or had serum creatinine (SCr) ≥1.5 mg/dL received 2.5 mg twice daily (n = 428). The study groups were well matched at baseline, with a mean age of 70 years and a mean CHADS2 score of 2.1. Pa- tient comorbidities included history of stroke or TIA (19%), hypertension (88%), diabetes (25%), heart failure (35.5%), history of myocardial infarc- tion (MI) (14%), and moderate or se- vere renal dysfunction (16.5%).
For those randomized to warfarin, the mean time spent in the therapeutic INR range of 2 to 3 was 66%. Rates of drug discontinuation were statistically higher for warfarin (27.5%) than for apixaban (25.3%), mostly attributed to a difference in the number of adverse drug events, though an equal propor- tion of discontinuations (44%) were due to adverse events or death for each group.
Apixaban was found to be superior to warfarin for the primary efficacy outcome of stroke or systemic embo- lism prevention, yielding a number needed to treat (NNT) of 303 for 1 year to prevent 1 event (1.27% vs 1.60%; P = 0.01). Likewise, apixaban was superior to warfarin for the pri- mary safety outcome of major bleed- ing (according to International Soci- ety on Thrombosis and Haemostasis [ISTH] criteria), with rates of 2.13% and 3.09% per year, respectively (P <0.001; NNT = 104).
Amid demonstration of superiority in clinical and safety end points, a com- bined net clinical outcome analysis was performed. Apixaban (6.13%) was su- perior to warfarin (7.20%) for the pre- vention of stroke, systemic embolism, death, or major bleeding, corresponding to an NNT of 93 (hazard ratio, 0.85; P <0.001).
Among pre-specified subgroups, no significant differences were observed for rates of stroke or systemic embolism, though P >0.10 was set as the level of significance for these interactions. Perti- nent subgroups included 2.5 mg or 5 mg apixaban twice daily versus warfarin. Although not statistically different from the 5-mg dose, the apixaban 2.5-mg dose versus warfarin confidence interval was wide and crossed 1.00; however, with only 831 patients enrolled in this dosing arm, it was likely underpowered to draw meaningful conclusions.
References
1. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in pa- tients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
2. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in non- valvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
3. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806-817.
COMMENTARY
A Potential Option for AF Patients
I
n order to understand the potential clinical implications of the ARISTOTLE trial, findings must be interpreted both alone and in light of available data supporting the use of other novel anticoagulants. The RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) and ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) trials evaluated other oral antico- agulants (dabigatran and rivaroxaban, respectively) for prevention of stroke or systemic embolism in patients with nonvalvular AF or flutter.1,2 Since a direct head-to- head comparison of these drugs has not been conducted and is unlikely, the trial designs, study populations, and results must be compared in order to determine the best therapeutic choice for a patient (pending FDA approval of apixaban, of course).
The study design of ARISTOTLE and the trial evaluating rivaroxaban in AF (ROCKET AF) were both double- blinded and randomized. However, the trial evaluating dabigatran in AF (RE-LY) was unblinded. The dabigatran trial did enroll a patient population that was more 14 Cardiology Review | February 2012 Anticoagulants similar to ARISTOTLE (similar age and baseline stroke risk), while the rivaroxaban study included patients of both greater age and risk of embolism. This difference is reflected in the higher rates of stroke and systemic embolism observed in ROCKET AF regardless of treatment assignment. Definitions for bleeding events were similar between trials.
Without direct comparison of these agents, and with differences in patient populations and study designs, it is hard to infer which agent would be superior. However, the results of ARISTOTLE showed apixaban to be superior with regard to both efficacy and safety. In the RE-LY trial, dabigatran 150 mg twice daily (the FDA-approved dose) was also found to be superior to warfarin for prevention of stroke, but this dose was associated with no difference in major bleeding. In ROCKET AF, no statis- tical difference was observed between rivaroxaban and warfarin for rates of stroke or systemic embolism (intent- to-treat population) or major bleeding. These differences may have been due to the higher stroke risk of patients enrolled in ROCKET AF, although no significant interaction based on CHADS2 subgroups was detected.
ARISTOTLE was the only trial to demonstrate a significant reduction in mortality and MI versus warfarin. Dabigatran 150 mg twice daily was associated with a nonsignificant lower rate of mortality but a significantly higher rate of MI than warfarin, while rivaroxaban demonstrated nonsignificant lower rates of both mortality and MI. On the other hand, both rivaroxaban and dabigatran 150 mg twice daily significantly lowered the rates of ischemic stroke versus warfarin, while the reduction with apixaban was nonsignificant. It is important to note that none of the trials was powered to detect differ- ences in these secondary outcomes. All 3 drugs significantly lowered the risk of intracranial bleeds compared with warfarin; however, dabigatran 150 mg twice daily and rivaroxaban were both associated with significantly higher rates of gastrointestinal bleeding.
Apixaban has an additional therapeutic niche based on the results of the AVERROES trial (Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fi- brillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment), which compared apixaban to aspirin for prevention of stroke and systemic embolic in patients with AF deemed unsuitable for Vitamin K antagonist therapy.3 In clinical practice, patients with AF considered unsuitable candidates for warfarin therapy, often due to concerns for bleeding, may be started on aspirin to provide some protection against embolic events.
This trial was stopped early due to the significant superiority of apixaban over aspirin in preventing embolic events. This benefit was demonstrated against both high (≥162 mg) and low-dose aspirin (<162 mg) subgroups. More significantly, rates of major bleeding were no different between apixaban and aspirin, though a nonsig- nificant higher rate of bleeding was observed with apixaban versus low-dose aspirin.
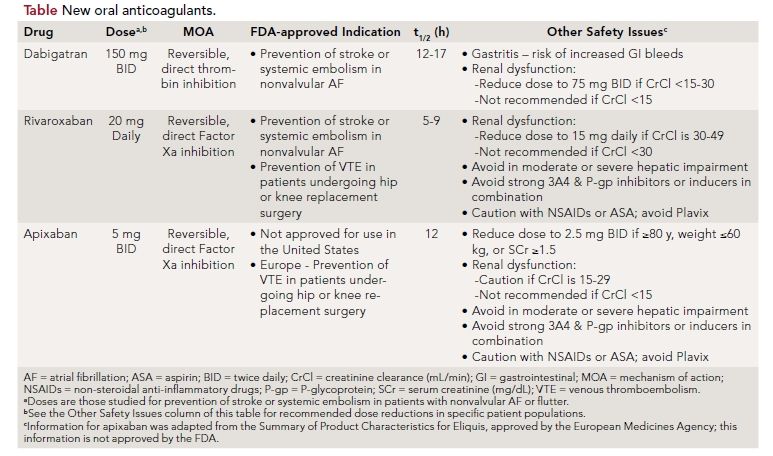
Despite the theoretical advantages of apixaban over dabigatran and rivaroxaban, it is not currently approved for use in the United States. Furthermore, while the lower doses of apixaban and rivaroxaban were studied in clinical trials for patients with renal dysfunction, the lower recommended dose of dabigatran (see the Table) in patients with renal dysfunction has not yet been stud- ied in comparison with warfarin. Other factors affecting drug selection are summarized in the Table. All three agents provide a lifestyle advantage over warfarin due to decreased clinic or doctor’s office visits for therapeutic monitoring as well as lack of dietary restrictions for patients. However, these agents will also be significantly more expensive than warfarin for years to come, and are not reversible in the setting of a bleed. Though they each have a shorter half-life than warfarin, no antidote comparable to Vitamin K for warfarin is available for any of these agents. Thus, switching to a newer agent may be unnecessary for patients whose INR is well controlled and who have tolerated warfarin therapy for years.
ARISTOTLE was the first trial to demonstrate the combined benefit of increased efficacy and safety versus warfarin and a significant decrease in mortality. Apixaban was superior to warfarin for the prevention of stroke or systemic embolism in patients with nonvalvular AF and also decreased rates of major bleeding and mortality. Pending FDA approval, it will represent yet another option for a patient population that has been committed to warfarin for many decades past.
About the Authors
Jennifer G. Bekker, PharmD, is a cardiology pharmacy Specialty Resident at University of Kentucky Health-Care Pharmacy Services and Jack and Linda Gill Heart Institute in Lexington, KY. She wrote this article in collaboration with Sara D. Brouse, PharmD, FCCP, BCPS-AQ Cardiology, Clinical Pharmacy Specialist Cardiology at University of Kentucky HealthCare Pharmacy Services and Jack and Linda Gill Heart Institute, Lexington, KY, and Editorial Board member Tracy e. MaCaulay, PharmD, BCPS-AQ Cardiology, Clinical Pharmacy Specialist-Cardiology, PGY2 Residency Program Director-Cardiology, and Adjunct Assistant Professor at University of Kentucky HealthCare, Department of Pharmacy Services and Division of Cardiovascular Medicine, Lexington, KY. February 2012
